Artificial Cascade Transformation Biosystem for One-Pot Biomanufacturing of Odd-Numbered Neoagarooligosaccharides and d-Tagatose through Wiser Agarose Utilization
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The application of agarose oligosaccharides has garnered great attention, with their biological activities varying among different structures. However, it still meets a great bottleneck for the targeted production of odd-numbered neoagarooligosaccharides (NAOSs), such as neoagarotriose (NA3), due to the lack of one-step hydrolases. In this work, the α-agarase AgaA33 and β-galactosidase BgaD were synergistically used to prepare NA3 with agarose as a substrate. Additionally, an l-arabinose isomerase CaLAI from Clostridium acetobutylicum was characterized to valorize low-value byproducts (d-galactose) by forming d-tagatose, which exhibited good thermal stability without the need for additional metal ions. Under the optimal reaction conditions, the production of NA3 and d-galactose catalyzed by these three enzymes was 0.40 and 0.15 g/L, respectively. The artificial three-enzyme-based cascade transformation system not only achieved the highest production of NA3 until now but also allowed for the valorization of d-galactose, providing a wiser application route for agarose utilization.
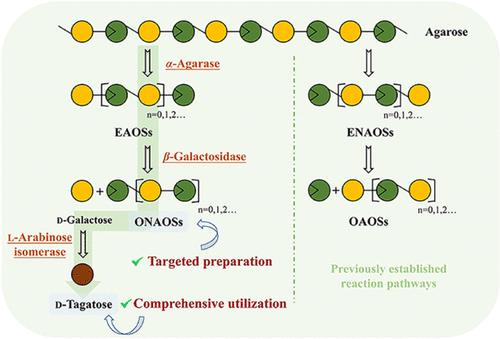
通过提高琼脂糖利用率实现奇数新戊寡糖和 d-塔格糖一锅生物制造的人工级联转化生物系统
琼脂糖寡糖的应用备受关注,不同结构的寡糖具有不同的生物活性。然而,由于缺乏一步水解酶,定向生产奇数新琼脂糖(NAOS)(如新琼脂三糖(NA3))仍面临巨大瓶颈。在这项工作中,α-琼脂糖酶 AgaA33 和 β-半乳糖苷酶 BgaD 被协同用于制备以琼脂糖为底物的 NA3。此外,来自乙酰丁酸梭菌(Clostridium acetobutylicum)的l-阿拉伯糖异构酶CaLAI通过形成d-塔格糖(d-galactose),实现了低价值副产物(d-半乳糖)的有价化,并表现出良好的热稳定性,无需额外的金属离子。在最佳反应条件下,这三种酶催化的 NA3 和 d-半乳糖产量分别为 0.40 和 0.15 克/升。基于人工三酶的级联转化系统不仅实现了迄今为止最高的 NA3 产量,还实现了 d-半乳糖的价值化,为琼脂糖的利用提供了一条更明智的应用途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


