Electrochemical Reactions Affected by Electric Double Layer Overlap in Conducting Nanopores
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
When a potential is applied to an electrode immersed in an electrolyte solution, ions with opposite charges accumulate around the electrode, forming an electrical double layer (EDL). Unlike flat electrodes, nanoporous electrodes with pore sizes comparable to the EDL thickness experience overlapping EDLs, altering the electrochemically effective surface area. Although previous research has primarily examined the ion charging dynamics and EDL formation in nanoporous electrodes, the impact of EDL overlap on Faraday reactions remains underexplored. In this study, we examined the influence of EDL overlap on electrochemical reactions within nanoporous electrodes using chronoamperometry and DC and AC voltammetry. We used the electrolyte concentration, measurement duration, overpotential, and electrode material as variables to determine the relationship between the extent of EDL overlap and the electrochemical reaction. The electrolyte concentration-dependent electrochemical reaction due to the EDL overlap was more pronounced for electrodes with faster potential changes, shorter measurement times, lower overpotentials, and slower catalytic activity. This is a unique nanoporous electrochemical phenomenon that is not observed on flat electrodes. These findings provide insight into the utilization of nanoporous electrodes in catalytic and sensor applications.
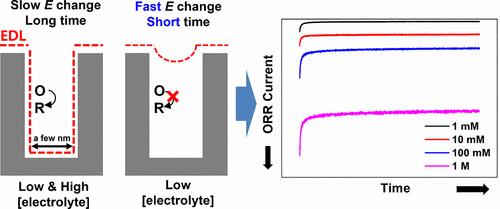
导电纳米孔中双电层重叠影响的电化学反应
当对浸入电解质溶液中的电极施加电位时,带相反电荷的离子会聚集在电极周围,形成电双层(EDL)。与平面电极不同,孔隙大小与 EDL 厚度相当的纳米多孔电极会出现 EDL 重叠,从而改变电化学有效表面积。虽然之前的研究主要考察了纳米多孔电极中的离子充电动力学和 EDL 形成,但 EDL 重叠对法拉第反应的影响仍未得到充分探索。在本研究中,我们使用计时电流计以及直流和交流伏安法研究了 EDL 重叠对纳米多孔电极内电化学反应的影响。我们使用电解质浓度、测量持续时间、过电位和电极材料作为变量来确定 EDL 重叠程度与电化学反应之间的关系。对于电位变化较快、测量时间较短、过电位较低和催化活性较慢的电极,EDL 重叠导致的电化学反应与电解质浓度有关的特性更为明显。这是一种独特的纳米多孔电化学现象,在平面电极上观察不到这种现象。这些发现为在催化和传感器应用中利用纳米多孔电极提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


