Exploring the cognitive effects of hearing loss in adult rats: Implications for visuospatial attention, social behavior, and prefrontal neural activity
IF 2.9
3区 医学
Q2 NEUROSCIENCES
引用次数: 0
Abstract
Age-related hearing loss in humans has been associated with cognitive decline, though the underlying mechanisms remain unknown. We investigated the long-term effects of hearing loss on attention, impulse control, social interaction, and neural activity within medial prefrontal cortex (mPFC) subregions.
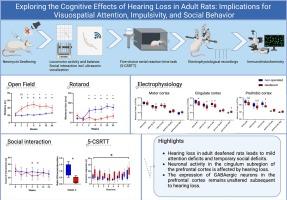
Hearing loss was induced in adult rats via intracochlear neomycin injection (n = 13), with non-operated rats as controls (n = 10). Rats were tested for motor activity (open field), coordination (Rotarod), and social interaction (including ultrasonic vocalization, USV) before surgery and at weeks 1, 2, 4, 8, 16, and 24 post-surgery. From week 8 on, rats were trained in the five-choice serial reaction time task (5-CSRTT) to assess visuospatial attention and impulse control. Finally, oscillatory neuronal activity in mPFC subregions was recorded with multielectrode arrays during anesthesia, followed by immunohistological staining for NeuN+ and Parvalbumin+ cells.
Deafened rats were more active than controls, whereas social interaction and USV were temporarily reduced. They also had difficulties to learn the concept of the 5-CSRTT paradigm and made more incorrect responses. Electrophysiology showed decreased power in theta, alpha, and beta frequency, and enhanced high gamma band in the mPFC in deafened rats, which was most pronounced in the cingulate subregion (Cg1). The number of NeuN+ and Parvalbumin+ cells, however, did not differ between groups.
The behavioral deficits together with the altered neuronal activity found in the Cg1 subregion of the mPFC in adult deafened rats may be used as an endophenotype to elucidate the mechanisms behind the cognitive decline seen in older patients with hearing loss.
探索听力损失对成年大鼠认知的影响:对视觉空间注意力、社交行为和前额叶神经活动的影响
人类与年龄相关的听力损失与认知能力下降有关,但其潜在机制仍不清楚。我们研究了听力损失对注意力、冲动控制、社会交往和内侧前额叶皮质(mPFC)亚区神经活动的长期影响。通过耳蜗内注射新霉素诱导成年大鼠听力损失(n = 13),以未手术大鼠为对照组(n = 10)。在手术前和手术后第 1、2、4、8、16 和 24 周,对大鼠进行了运动活动(空场)、协调(旋转)和社会交往(包括超声发声)测试。从第8周开始,对大鼠进行五选一连续反应时间任务(5-CSRTT)训练,以评估视觉空间注意力和冲动控制能力。最后,在麻醉状态下使用多电极阵列记录 mPFC 亚区域的振荡神经元活动,然后对 NeuN+ 和 Parvalbumin+ 细胞进行免疫组织学染色。聋鼠比对照组更活跃,但社会交往和USV暂时减少。它们也很难学会5-CSRTT范式的概念,并做出更多错误的反应。电生理学研究显示,耳聋大鼠的 mPFC 中θ、α和β频率的功率下降,高γ波段增强,这在扣带回亚区(Cg1)最为明显。然而,NeuN+和Parvalbumin+细胞的数量在不同组间并无差异。在成年耳聋大鼠的 mPFC Cg1 亚区发现的行为缺陷和神经元活动改变可作为一种内表型,用于阐明老年听力损失患者认知能力下降的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuroscience
医学-神经科学
CiteScore
6.20
自引率
0.00%
发文量
394
审稿时长
52 days
期刊介绍:
Neuroscience publishes papers describing the results of original research on any aspect of the scientific study of the nervous system. Any paper, however short, will be considered for publication provided that it reports significant, new and carefully confirmed findings with full experimental details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


