High-Yield Bioproduction of Extracellular Vesicles from Stem Cell Spheroids via Millifluidic Vortex Transport
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
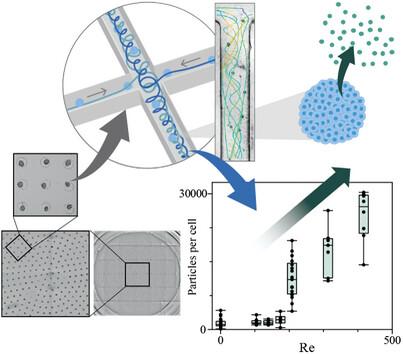
Extracellular vesicles (EVs) are emerging as novel therapeutics, particularly in cancer and degenerative diseases. Nevertheless, from both market and clinical viewpoints, high-yield production methods using minimal cell materials are still needed. Herein, a millifluidic cross-slot chip is proposed to induce high-yield release of biologically active EVs from less than three million cells. Depending on the flow rate, a single vortex forms in the outlet channels, exposing transported cellular material to high viscous stresses. Importantly, the chip accommodates producer cells within their physiological environment, such as human mesenchymal stem cells (hMSCs) spheroids, while facilitating their visualization and individual tracking within the vortex. This precise control of viscous stresses at the spheroid level allows for the release of up to 30000 EVs per cell at a Reynolds number of ≈400, without compromising cellular integrity. Additionally, it reveals a threshold initiating EV production, providing evidence for a stress-dependent mechanism governing vesicle secretion. EVs mass-produced at high Reynolds displayed pro-angiogenic and wound healing capabilities, as confirmed by proteomic and cytometric analysis of their cargo. These distinct molecular signatures of these EVs, compared to those derived from monolayers, underscore the critical roles of the production method and the 3D cellular environment in EV generation.
通过毫流体涡旋输送从干细胞球体中高产生物生产细胞外囊泡
细胞外囊泡(EVs)正在成为一种新型疗法,尤其是在癌症和退行性疾病方面。然而,从市场和临床角度来看,仍需要使用最少细胞材料的高产生产方法。在此,我们提出了一种毫米级流体交叉槽芯片,可从不到三百万个细胞中诱导生物活性 EVs 的高产释放。根据流速的不同,出口通道中会形成单涡流,使输送的细胞材料暴露在高粘性应力下。重要的是,该芯片可容纳处于生理环境中的生产者细胞,如人类间充质干细胞(hMSCs)球形体,同时便于在涡流中对它们进行可视化和单独跟踪。这种在球体水平上对粘性应力的精确控制,可使每个细胞在雷诺数≈400的条件下释放多达30000个EV,而不会损害细胞的完整性。此外,它还揭示了启动EV产生的阈值,为一种依赖于应力的囊泡分泌机制提供了证据。在高雷诺条件下大量生产的EV具有促血管生成和伤口愈合的能力,这一点已通过对其货物的蛋白质组学和细胞计量学分析得到证实。与来自单层细胞的EV相比,这些EV具有不同的分子特征,突出了生产方法和三维细胞环境在EV生成中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


