Boosting the Structural and Electrochemical Stability of Chloride-Ion-Conducting Perovskite Solid Electrolytes by Alkali Ion Doping
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The use of chloride-based solid electrolytes derived from Lewis acid‒base reactions enables the construction of various new rechargeable batteries, such as chloride ion batteries (CIBs). However, a critical problem with these electrolytes is their poor stability under low-temperature, moist, or electrochemical conditions, which can lead to deterioration of the phase structure and a loss of ion conduction. Herein, the robust cubic structure of tin-based perovskite chloride—a chloride ion conductor—is achieved by alkali ion doping at the tin site via direct mechanical milling. The as-prepared cubic CsSn0.925Na0.075Cl2.925 (CSNC) electrolyte exhibits outstanding structural stability over a broad temperature range of 213−473 K or under a high relative humidity of up to 90%, at which the typical chloride electrolytes previously reported deteriorate because of moisture. Importantly, mild annealing can modify the microstructure of the CSNC, resulting in a two fold increase in ionic conductivity and an increase in electrochemical stability, which is superior to those of other chloride electrolytes reported in previous studies. The effective chloride-ion transfer and wide electrochemical window of the CSNC are further demonstrated in different solid-state CIBs.
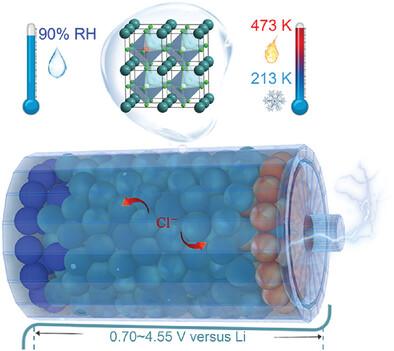
通过掺入碱离子提高氯离子导电包晶固体电解质的结构和电化学稳定性
使用从路易斯酸碱反应中提取的氯基固体电解质可以制造出各种新型可充电电池,如氯离子电池(CIB)。然而,这些电解质的一个关键问题是在低温、潮湿或电化学条件下稳定性差,可能导致相结构退化和离子传导能力下降。在本文中,通过直接机械研磨法在锡位点掺入碱离子,实现了锡基氯化透辉石--一种氯离子导体--的稳健立方结构。所制备的立方体 CsSn0.925Na0.075Cl2.925 (CSNC) 电解质在 213-473 K 的宽温度范围或高达 90% 的高相对湿度条件下均表现出出色的结构稳定性,而之前报道的典型氯化物电解质在这种条件下会因受潮而变质。重要的是,温和退火可以改变 CSNC 的微观结构,从而使离子电导率和电化学稳定性提高两倍,优于以往研究中报道的其他氯化物电解质。CSNC 的有效氯离子转移和宽电化学窗口在不同的固态 CIB 中得到了进一步证实。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


