Ferritinophagy is involved in hexavalent chromium-induced ferroptosis in Sertoli cells
IF 3.3
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Hexavalent chromium [Cr(VI)] has significant adverse effects on the environment and human health, particularly on the male reproductive system. Previously, we observed ferroptosis and autophagy in rat testicular injury induced by Cr(VI). In the present study, we focused on the association between ferroptosis and autophagy in mouse Sertoli cells (TM4) exposed to concentrations of 2.5 μМ, 5 μМ, and 10 μМ Cr(VI). Cr(VI) exposure altered mitochondrial ultrastructure; increased intracellular iron, malondialdehyde, and reactive oxygen species (ROS) levels; decreased glutathione content; increased TfR1 protein expression; and decreased GPX4, FPN1, and SLC7A11 protein expression, ultimately resulting in ferroptosis. Additionally, we observed ferritinophagy, increased expression of BECLIN1, LC3B, and NCOA4, and decreased expression of FTH1 and P62. Inhibition of autophagy and ferritinophagy via 3-MA and small interfering RNA (siRNA)-mediated silencing of NCOA4 ameliorated changes in ferritinophagy- and ferroptosis-associated protein expression, and reduced ROS levels. Rats exposed to Cr(VI) exhibited atrophy of testicular seminiferous tubules, a reduction in germ and Sertoli cells, and the occurrence of ferritinophagy and ferroptosis in cells of the rat testes. These results indicate that ferroptosis, triggered by NCOA4-mediated ferritinophagy, is one of the mechanisms that contribute to Cr(VI)-induced damage in Sertoli cells.
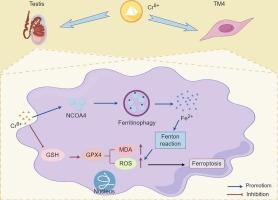
铁蛋白吞噬参与了六价铬诱导的 Sertoli 细胞铁突变。
六价铬[Cr(VI)]对环境和人类健康,尤其是对男性生殖系统有重大不利影响。此前,我们曾在六价铬诱导的大鼠睾丸损伤中观察到铁蛋白沉着和自噬现象。在本研究中,我们重点研究了暴露于 2.5 μМ、5 μМ和 10 μМ六价铬的小鼠 Sertoli 细胞(TM4)中铁细胞沉降和自噬之间的关联。铬(Ⅵ)暴露改变了线粒体的超微结构;增加了细胞内铁、丙二醛和活性氧(ROS)的含量;降低了谷胱甘肽的含量;增加了 TfR1 蛋白的表达;降低了 GPX4、FPN1 和 SLC7A11 蛋白的表达,最终导致了铁变态反应。此外,我们还观察到铁蛋白吞噬,BECLIN1、LC3 和 NCOA4 表达增加,FTH1 和 P62 表达减少。通过 3-MA 和小干扰 RNA(siRNA)介导的 NCOA4 沉默抑制自噬和噬铁蛋白,可改善噬铁蛋白和铁突变相关蛋白表达的变化,并降低 ROS 水平。暴露于六价铬的大鼠表现出睾丸曲细精管萎缩、生精细胞和Sertoli细胞减少、嗜铁蛋白和铁突变的发生。这些结果表明,由 NCOA4 介导的噬铁蛋白引发的铁蛋白沉积是导致铬(VI)诱导的 Sertoli 细胞损伤的机制之一。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


