Neonatal hypoxia-ischemia alters the events governing the hippocampal critical period of postnatal synaptic plasticity leading to deficits in working memory in mice
IF 5.1
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Postnatal critical periods of synaptic plasticity (CPsp) are characterized by profound neural network refinement, which is shaped by synaptic activity and sculpted by maturation of the GABAergic network. Even after therapeutic hypothermia (TH), neonatal hypoxia-ischemia (HI) impairs two triggers for the initiation of the CPsp in the hippocampus: i) PSA-NCAM developmental decline and ii) parvalbumin (PV) + interneuron (IN) maturation. Thus, we investigated whether neonatal HI despite TH disturbs other events governing the onset, consolidation and closure of the postnatal CPsp in the hippocampus. We induced cerebral HI in P10 C57BL6 mice with right carotid ligation and 45 m of hypoxia (FiO2 = 0.08), followed by normothermia (36 °C, NT) or TH (31 °C) for 4 h with anesthesia-exposed shams as controls. ELISA, immunoblotting and immunohistochemistry were performed at 24 h (P11), 5 days (P15), 8 days (P18) and 30 days (P40) after HI injury. We specifically assessed: i) BDNF levels and TrkB activation, controlling the CPsp, ii) Otx2 and NPTX2 immunoreactivity (IR), engaging CPsp onset and iii) NogoR1, Lynx1 IR, PNN formation and myelination (MBP) mediating CPsp closure. Pups aged to P40 also received a battery of tests assessing working memory. Here, we documented deficits in hippocampal BDNF levels and TrkB activation at P18 in response to neonatal HI even with TH. Neonatal HI impaired in the CA1 the developmental increase in PV, Otx2, and NPTX2 between P11 and P18, the colocalization of Otx2 and PV at P18 and P40, the accumulation of NPTX2 in PV+ dendrites at P18 and P40, and the expression of NogoR at P40. Furthermore, neonatal HI decreased BDNF and impaired PNN development and myelination (MBP) at P40. Most of these abnormalities were insensitive to TH and correlated with memory deficits. Neonatal HI appears to disrupt many of the molecular and structural events initiating and consolidating the postnatal hippocampal CPsp, perhaps due to the early and delayed deficits in TrkB activation leading to memory deficits.
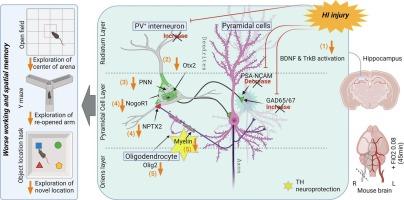
新生儿缺氧缺血会改变海马出生后突触可塑性关键期的事件,导致小鼠工作记忆缺陷。
出生后突触可塑性关键期(CPsp)的特点是神经网络的深度完善,它由突触活动塑造,并由 GABA 能网络的成熟雕刻而成。即使在治疗性低温(TH)之后,新生儿缺氧缺血(HI)也会损害海马中启动 CPsp 的两个触发因素:i)PSA-NCAM 发育衰退和 ii)副发光体(PV)+中间神经元(IN)成熟。因此,我们研究了新生儿HI(尽管有TH)是否会干扰海马出生后CPsp的发生、巩固和关闭的其他事件。我们用右颈动脉结扎和 45 米低氧(FiO2 = 0.08)诱导 P10 C57BL6 小鼠脑 HI,然后进行常温(36 °C,NT)或 TH(31 °C)4 小时,以麻醉暴露的假体作为对照。分别在 HI 损伤后 24 小时(P11)、5 天(P15)、8 天(P18)和 30 天(P40)进行 ELISA、免疫印迹和免疫组织化学检测。我们特别评估了:i)控制 CPsp 的 BDNF 水平和 TrkB 激活;ii)参与 CPsp 发生的 Otx2 和 NPTX2 免疫反应(IR);iii)介导 CPsp 闭合的 NogoR1、Lynx1 IR、PNN 形成和髓鞘化(MBP)。年龄达到 P40 的幼犬还接受了一系列评估工作记忆的测试。在这里,我们记录了海马 BDNF 水平和 TrkB 激活在 P18 时对新生儿 HI(即使有 TH)的反应缺陷。新生儿 HI 影响了 CA1 中 PV、Otx2 和 NPTX2 在 P11 和 P18 之间的发育增加,Otx2 和 PV 在 P18 和 P40 时的共定位,NPTX2 在 P18 和 P40 时在 PV+ 树突中的积累,以及 NogoR 和 Lynx1 在 P40 时的表达。此外,新生儿 HI 会降低 BDNF,并在 P40 时损害 PNN 的发育和髓鞘化(MBP)。这些异常大多对 TH 不敏感,并与记忆缺陷相关。新生儿 HI 似乎破坏了许多启动和巩固出生后海马 CPsp 的分子和结构事件,这可能是由于 TrkB 激活的早期和延迟缺陷导致了记忆缺陷。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurobiology of Disease
医学-神经科学
CiteScore
11.20
自引率
3.30%
发文量
270
审稿时长
76 days
期刊介绍:
Neurobiology of Disease is a major international journal at the interface between basic and clinical neuroscience. The journal provides a forum for the publication of top quality research papers on: molecular and cellular definitions of disease mechanisms, the neural systems and underpinning behavioral disorders, the genetics of inherited neurological and psychiatric diseases, nervous system aging, and findings relevant to the development of new therapies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


