Mechanotransductive N-cadherin binding induces differentiation in human neural stem cells
引用次数: 0
Abstract
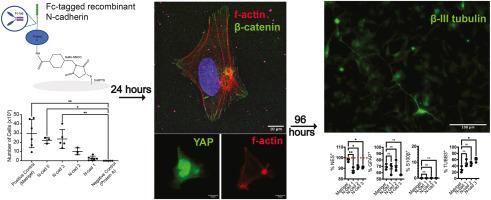
The neural stem cell niche is a complex microenvironment that includes cellular factors, secreted factors, and physical factors that impact stem cell behavior and development. Cellular interactions through cadherins, cell–cell binding proteins, have implications in embryonic development and mesenchymal stem cell differentiation. However, little is known about the influence of cadherins within the neural stem cell microenvironment and their effect on human stem cell maintenance and differentiation. Therefore, the purpose of this study was to develop synthetic substrates to examine the effect of cadherin mechanotransduction on human neural stem cells. Glass substrates were fabricated using silane, protein A, and recombinant N-cadherin; we used these substrates to examine the effect of N-cadherin binding on neural stem cell proliferation, cytoskeletal structure and morphology, Yes-associated protein-1 (YAP) translocation, and differentiation. Bound exogenous N-cadherin induced concentration-dependent increases in adherens junction formation, YAP translocation, and early expression of neurogenic differentiation markers. Strong F-actin ring structures were initiated by homophilic N-cadherin binding, eliciting neuronal differentiation of cells within 96 h without added soluble differentiation factors. Our findings show that active N-cadherin binding plays an important role for differentiation of human iPS-derived neural stem cells towards neurons, providing a new tool to differentiate cells in vitro.
机械传导性 N-粘连蛋白结合诱导人类神经干细胞分化
神经干细胞龛是一种复杂的微环境,包括影响干细胞行为和发育的细胞因子、分泌因子和物理因子。通过粘附蛋白(细胞-细胞结合蛋白)进行的细胞相互作用对胚胎发育和间充质干细胞分化有影响。然而,人们对神经干细胞微环境中的粘附蛋白的影响及其对人类干细胞维持和分化的作用知之甚少。因此,本研究的目的是开发合成基底,以研究固着蛋白机械传导对人类神经干细胞的影响。我们使用硅烷、蛋白A和重组N-粘连蛋白制作了玻璃基底,并利用这些基底研究了N-粘连蛋白结合对神经干细胞增殖、细胞骨架结构和形态、Yes相关蛋白-1(YAP)转位和分化的影响。结合外源N-cadherin可诱导粘连接头形成、YAP转位和神经原分化标记早期表达的浓度依赖性增加。同亲和的N-cadherin结合启动了强大的F-actin环结构,在96小时内激发了细胞的神经元分化,而无需添加可溶性分化因子。我们的研究结果表明,活跃的N-cadherin结合在人类iPS神经干细胞向神经元分化的过程中发挥了重要作用,为体外细胞分化提供了一种新工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


