Evidence for the Quercetin Binding Site of Glycogen Phosphorylase as a Target for Liver-Isoform-Selective Inhibitors against Glioblastoma: Investigation of Flavanols Epigallocatechin Gallate and Epigallocatechin
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Glycogen phosphorylase (GP) is the rate-determining enzyme in glycogenolysis, and its druggability has been extensively studied over the years for the development of therapeutics against type 2 diabetes (T2D) and, more recently, cancer. However, the conservation of binding sites between the liver and muscle isoforms makes the inhibitor selectivity challenging. Using a combination of kinetic, crystallographic, modeling, and cellular studies, we have probed the binding of dietary flavonoids epigallocatechin gallate (EGCG) and epigallocatechin (EGC) to GP isoforms. The structures of rmGPb-EGCG and rmGPb-EGC complexes were determined by X-ray crystallography, showing binding at the quercetin binding site (QBS) in agreement with kinetic studies that revealed both compounds as noncompetitive inhibitors of GP, with EGCG also causing a significant reduction in cell viability and migration of U87-MG glioblastoma cells. Interestingly, EGCG exhibits different binding modes to GP isoforms, revealing QBS as a promising site for GP targeting, offering new opportunities for the design of liver-selective GP inhibitors.
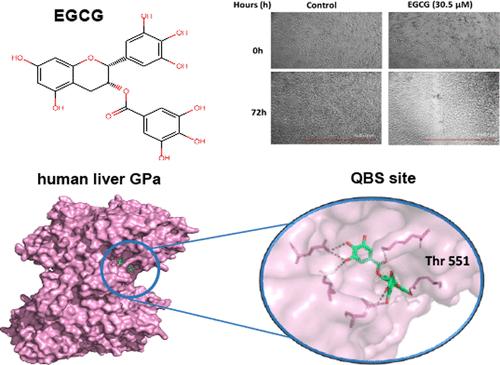
证明糖原磷酸化酶的槲皮素结合位点是针对胶质母细胞瘤的肝异构体选择性抑制剂的靶点:对黄烷醇表没食子儿茶素没食子酸酯和表没食子儿茶素的研究
糖原磷酸化酶(GP)是糖原分解过程中的速率决定酶,多年来,人们一直在广泛研究它的可药性,以开发针对 2 型糖尿病(T2D)和癌症的疗法。然而,由于肝脏和肌肉同工酶之间的结合位点保持不变,因此抑制剂的选择性具有挑战性。我们采用动力学、晶体学、建模和细胞研究相结合的方法,探究了膳食类黄酮表没食子儿茶素没食子酸酯(EGCG)和表没食子儿茶素(EGC)与 GP 同工酶的结合。X射线晶体学测定了rmGPb-EGCG和rmGPb-EGC复合物的结构,结果表明它们在槲皮素结合位点(QBS)结合,这与动力学研究一致,动力学研究显示这两种化合物都是GP的非竞争性抑制剂,EGCG还能显著降低U87-MG胶质母细胞瘤细胞的活力和迁移。有趣的是,EGCG 表现出与 GP 异构体不同的结合模式,揭示了 QBS 是 GP 靶向的一个有希望的位点,为设计肝脏选择性 GP 抑制剂提供了新的机遇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


