Kiwifruit Polysaccharides Alleviate Ulcerative Colitis via Regulating Gut Microbiota-Dependent Tryptophan Metabolism and Promoting Colon Fucosylation
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
A previous study showed that kiwifruit polysaccharide (KFP) has benefits in relieving intestinal inflammation, while the underlying mechanism remains unresolved. The objective of this study was to investigate the regulatory effect of KFP on the gut microbiota metabolism and intestinal barrier of ulcerative colitis (UC) mice induced by dextran sulfate sodium (DSS). KFP significantly improved the UC symptoms including weight loss, shortened colon length, splenomegaly, diarrhea, hematochezia, and colon inflammation of mice. In addition, KFP could alleviate DSS-caused gut microbiota dysbiosis and increase the levels of short-chain fatty acids in the cecal contents of mice. Furthermore, the results of nontargeted and targeted metabolomics analysis combined with antibiotic treatment revealed that KFP could regulate gut microbiota-dependent tryptophan metabolism, activate the aryl hydrocarbon receptor (AhR) in colon cells, and enhance interleukin-22 production and tight junction proteins' (ZO-1, occludin, and claudin3) expression to repair the intestinal barrier in UC mice. Immunofluorescence results showed that KFP significantly upregulated the conjunction of lectin WGA and UEA1 in the UC mouse colon, implying that KFP promoted fucosylation in the colon. These results suggest that KFP alleviates UC primarily via targeting the gut microbiota involved in the AhR pathway and upregulating colon fucosylation.
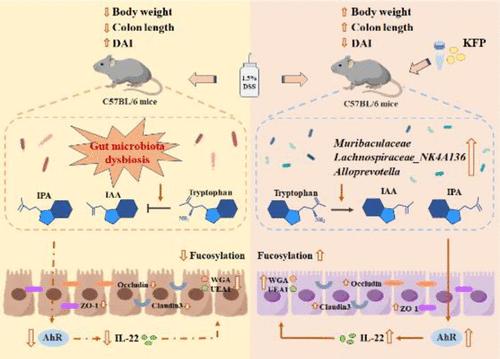
猕猴桃多糖通过调节肠道微生物群依赖性色氨酸代谢和促进结肠岩藻糖基化缓解溃疡性结肠炎
先前的一项研究表明,猕猴桃多糖(KFP)具有缓解肠道炎症的功效,但其潜在机制仍未得到解决。本研究旨在探讨猕猴桃多糖对葡聚糖硫酸钠(DSS)诱导的溃疡性结肠炎(UC)小鼠肠道微生物群代谢和肠道屏障的调节作用。KFP能明显改善小鼠的溃疡性结肠炎症状,包括体重减轻、结肠长度缩短、脾肿大、腹泻、血尿和结肠炎症。此外,KFP 还能缓解 DSS 引起的肠道微生物群失调,提高小鼠盲肠内容物中短链脂肪酸的水平。此外,结合抗生素治疗进行的非靶向和靶向代谢组学分析结果显示,KFP能调节肠道微生物群依赖性色氨酸代谢,激活结肠细胞中的芳基烃受体(AhR),并增强白细胞介素-22的产生和紧密连接蛋白(ZO-1、occludin和claudin3)的表达,从而修复UC小鼠的肠道屏障。免疫荧光结果显示,KFP能显著上调UC小鼠结肠中凝集素WGA和UEA1的连接,这意味着KFP促进了结肠中的岩藻糖基化。这些结果表明,KFP 主要通过靶向参与 AhR 通路的肠道微生物群和上调结肠岩藻糖基化来缓解 UC。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


