Supplementation of spermidine at 40 mg/day has minimal effects on circulating polyamines: An exploratory double-blind randomized controlled trial in older men
IF 3.4
3区 医学
Q2 NUTRITION & DIETETICS
引用次数: 0
Abstract
This study represents the first investigation into the safety of a novel, high-purity spermidine trihydrochloride supplement (hpSPD) in humans. Spermidine, a natural compound found in various foods, has demonstrated potential health benefits in animal and epidemiological studies. However, evidence from clinical trials and safety evaluations of spermidine supplements is limited because pure spermidine for human administration has not been available. In this randomized, double-blind, within-subject and placebo-controlled trial, 37 healthy men (age 50–70 years; body mass index, 18.5–28 kg/m2) were administered either hpSPD or a placebo. We hypothesized that 7-day and 28-day dosing of 40 mg/day of hpSPD would have minimal effects on safety, although metabolic and polyamine homeostasis has not previously been examined at this dosage level. Consistent with our hypothesis, 40 mg/day hpSPD did not result in any significant changes in clinical, lipids, chemistry, or hematological parameters compared to placebo. Compliance was high, and no study product-related adverse events were reported. Substantial changes in serum and urine polyamine concentrations were not observed following hpSPD supplementation, suggesting effective homeostatic control of full-dose highly purified spermidine supplements with no evidence of adaptation of spermidine metabolism at 40 mg/day. These findings suggest that hpSPD at 40 mg/day for up to 28 days is safe and well-tolerated in healthy older men. The study is consistent with preclinical results and provides important evidence supporting the safety of high-purity spermidine supplementation, enabling further research with single-molecule spermidine to investigate its potential biology for improving human health. This trial was registered at clinicaltrials.gov (NCT05459961).
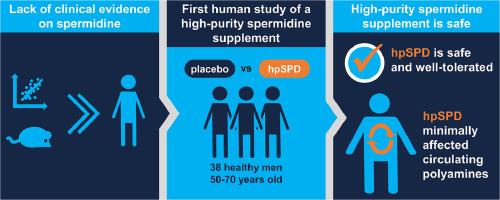
每天补充 40 毫克的亚精胺对循环多胺的影响微乎其微:一项针对老年男性的探索性双盲随机对照试验
这项研究首次调查了一种新型高纯度三盐酸精胺补充剂(hpSPD)对人体的安全性。精脒是一种存在于各种食物中的天然化合物,在动物和流行病学研究中已被证明具有潜在的健康益处。然而,由于还没有供人类服用的纯品精胺,因此精胺补充剂的临床试验和安全性评估证据非常有限。在这项随机、双盲、受试者内和安慰剂对照试验中,37 名健康男性(年龄 50-70 岁;体重指数 18.5-28 kg/m2)服用了 hpSPD 或安慰剂。我们的假设是,每天服用 40 毫克 hpSPD 的 7 天和 28 天剂量对安全性的影响极小,尽管此前尚未在这一剂量水平上对代谢和多胺稳态进行过研究。与我们的假设一致,与安慰剂相比,40 毫克/天的 hpSPD 不会导致临床、血脂、化学或血液学参数发生任何显著变化。患者的依从性很高,也未报告与研究产品相关的不良事件。补充hpSPD后,血清和尿液中的多胺浓度没有发生重大变化,这表明全剂量高纯度亚精胺补充剂能有效控制体内平衡,没有证据表明服用40毫克/天的亚精胺代谢会发生适应性变化。这些研究结果表明,健康老年男性每天服用 40 毫克的 hpSPD 长达 28 天是安全和耐受性良好的。该研究与临床前研究结果一致,并提供了支持高纯度亚精胺补充剂安全性的重要证据,有助于进一步研究单分子亚精胺,探讨其在改善人类健康方面的潜在生物学作用。该试验已在 clinicaltrials.gov 注册(NCT05459961)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nutrition Research
医学-营养学
CiteScore
7.60
自引率
2.20%
发文量
107
审稿时长
58 days
期刊介绍:
Nutrition Research publishes original research articles, communications, and reviews on basic and applied nutrition. The mission of Nutrition Research is to serve as the journal for global communication of nutrition and life sciences research on diet and health. The field of nutrition sciences includes, but is not limited to, the study of nutrients during growth, reproduction, aging, health, and disease.
Articles covering basic and applied research on all aspects of nutrition sciences are encouraged, including: nutritional biochemistry and metabolism; metabolomics, nutrient gene interactions; nutrient requirements for health; nutrition and disease; digestion and absorption; nutritional anthropology; epidemiology; the influence of socioeconomic and cultural factors on nutrition of the individual and the community; the impact of nutrient intake on disease response and behavior; the consequences of nutritional deficiency on growth and development, endocrine and nervous systems, and immunity; nutrition and gut microbiota; food intolerance and allergy; nutrient drug interactions; nutrition and aging; nutrition and cancer; obesity; diabetes; and intervention programs.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


