Intestinal-level anti-inflammatory bioactivities of whole wheat: Rationale, design, and methods of a randomized, controlled, crossover dietary trial in adults with prediabetes
IF 3.4
3区 医学
Q2 NUTRITION & DIETETICS
引用次数: 0
Abstract
Randomized controlled trials (RCT) demonstrate that whole wheat consumption improves glycemia. However, substantial inter-individual variation is often observed, highlighting that dietary whole grain recommendations may not support the health of all persons. The objective of this report is to describe the rationale and design of a planned RCT aimed at establishing the gut microbiota and metabolome signatures that predict whole wheat-mediated improvements in glucose tolerance in adults with prediabetes. It is hypothesized that a controlled diet containing wheat bread (WHEAT; 160 g/day) compared with refined bread (WHITE) will improve glucose tolerance in a gut microbiota-mediated manner. Biospecimens will be collected before and after each 2-week study arm. Testing for oral glucose tolerance and gastrointestinal permeability will be performed post-intervention. Assessments will include oral glucose tolerance (primary outcome) and secondary outcomes including gut microbiota, targeted and untargeted metabolomics of fecal and plasma samples, intestinal and host inflammatory responses, and intestinal permeability. WHEAT is predicted to alleviate glucose intolerance by shifting microbiota composition to increase short-chain fatty acid-producing bacteria while reducing populations implicated in intestinal inflammation, barrier dysfunction, and systemic endotoxemia. Further, benefits from WHEAT are anticipated to correlate with gut-level and systemic metabolomic responses that can help to explain the expected inter-individual variability in glucose tolerance. Thus, knowledge gained from integrating multi-omic responses associating with glucose tolerance could help to establish a precision nutrition-based framework that can alleviate cardiometabolic risk. This framework could inform novel dietary whole grain recommendations by enhancing our understanding of inter-individual responsiveness to whole grain consumption.
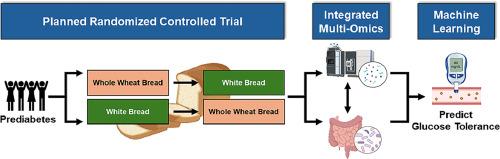
全麦的肠道级抗炎生物活性:糖尿病前期成人随机对照交叉膳食试验的原理、设计和方法。
随机对照试验(RCT)表明,食用全麦可改善血糖。然而,个体之间往往存在很大差异,这突出表明全谷物膳食建议可能无法支持所有人的健康。本报告旨在描述一项计划中的 RCT 的原理和设计,该 RCT 旨在确定肠道微生物群和代谢组特征,以预测全麦介导的糖尿病前期成人葡萄糖耐量改善情况。假设与精制面包(白面包)相比,控制饮食中含有小麦面包(WHEAT;160 克/天)将通过肠道微生物群介导的方式改善葡萄糖耐量。将在每个为期两周的研究臂之前和之后采集生物样本。干预后将进行口服葡萄糖耐量和胃肠道渗透性测试。评估将包括口服葡萄糖耐量(主要结果)和次要结果,包括肠道微生物群、粪便和血浆样本的靶向和非靶向代谢组学、肠道和宿主炎症反应以及肠道渗透性。据预测,WHEAT 可通过改变微生物群的组成来增加短链脂肪酸细菌的数量,同时减少与肠道炎症、屏障功能障碍和全身性内毒素血症有关的细菌数量,从而缓解葡萄糖不耐受症。此外,WHEAT 的益处预计与肠道和全身代谢组学反应相关,有助于解释葡萄糖耐量的预期个体间差异。因此,整合与葡萄糖耐量相关的多组反应所获得的知识有助于建立一个基于精准营养的框架,从而减轻心脏代谢风险。通过加强我们对全谷物消费的个体间反应的了解,这一框架可为新的全谷物膳食建议提供依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nutrition Research
医学-营养学
CiteScore
7.60
自引率
2.20%
发文量
107
审稿时长
58 days
期刊介绍:
Nutrition Research publishes original research articles, communications, and reviews on basic and applied nutrition. The mission of Nutrition Research is to serve as the journal for global communication of nutrition and life sciences research on diet and health. The field of nutrition sciences includes, but is not limited to, the study of nutrients during growth, reproduction, aging, health, and disease.
Articles covering basic and applied research on all aspects of nutrition sciences are encouraged, including: nutritional biochemistry and metabolism; metabolomics, nutrient gene interactions; nutrient requirements for health; nutrition and disease; digestion and absorption; nutritional anthropology; epidemiology; the influence of socioeconomic and cultural factors on nutrition of the individual and the community; the impact of nutrient intake on disease response and behavior; the consequences of nutritional deficiency on growth and development, endocrine and nervous systems, and immunity; nutrition and gut microbiota; food intolerance and allergy; nutrient drug interactions; nutrition and aging; nutrition and cancer; obesity; diabetes; and intervention programs.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


