Insight into the enhancement and mechanism of saltiness perception by salty peptides from bovine bone
IF 8.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Food-derived salty peptides have been considered promising substitutes for sodium salt. In this work, three novel salty dipeptides Asp-Pro (DP), Asp-Arg (DR), and Arg-Glu (RE) were identified from bovine bone hydrolysates. The salt reduction rates were 76.85 %, 77.28 %, and 73.72 % by the three peptides (2 mg/mL) in a NaCl concentration of 0.203 g/100 mL, respectively. According to Stevens' law, a non-linear relationship between saltiness intensity and concentration was quantified, showing a slower increase in the sensory intensity perception compared with the changes in physical concentration (β < 1). In molecular detail, electrostatic energy and van der Waals energy were the main energetic contributions to forming stable complexes. The binding of salty peptides to TMC4 was driven by hydrogen bonding and salt bridge, and the main binding sites were Glu319, Ala579, and Thr581. These results could provide new insight into the salt-enhancing property and interaction mechanism of salty peptides as novel sodium substitutes.

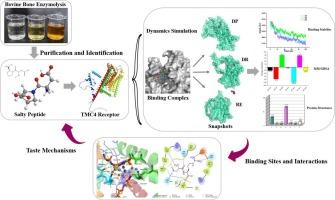
牛骨中的咸味肽对咸味感知能力的增强及其机制的启示
从食物中提取的咸肽一直被认为是钠盐的理想替代品。这项研究从牛骨水解物中发现了三种新型咸味二肽:Asp-Pro(DP)、Asp-Arg(DR)和Arg-Glu(RE)。在氯化钠浓度为 0.203 克/100 毫升时,这三种肽(2 毫克/毫升)的减盐率分别为 76.85%、77.28% 和 73.72%。根据史蒂文斯定律,咸味强度与浓度之间存在非线性关系,与物理浓度的变化相比,咸味强度的增加速度较慢(β <1)。从分子细节来看,静电能和范德华能是形成稳定复合物的主要能量贡献。咸肽与 TMC4 的结合是由氢键和盐桥驱动的,主要结合位点是 Glu319、Ala579 和 Thr581。这些结果为了解咸肽作为新型钠替代物的增盐特性和相互作用机制提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


