RNA granules in flux: dynamics to balance physiology and pathology
IF 26.7
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
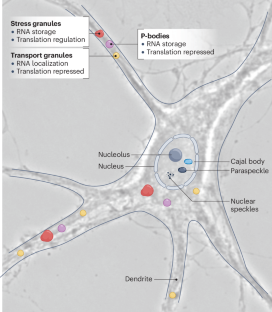
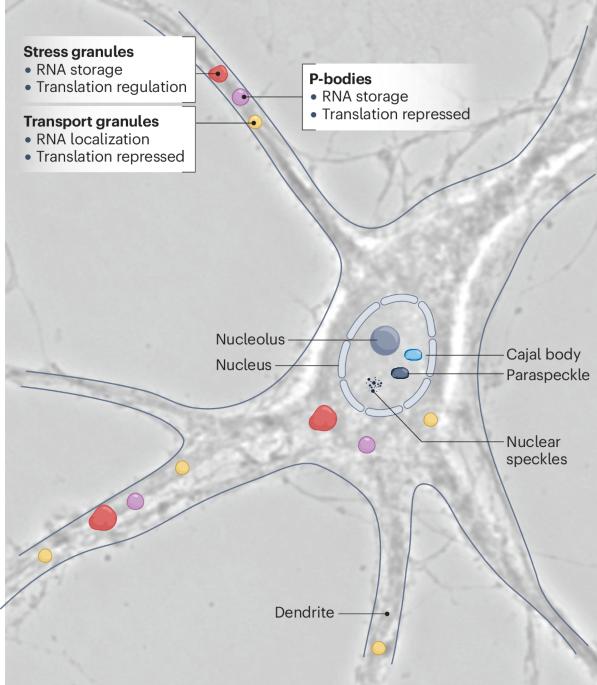
The life cycle of an mRNA is a complex process that is tightly regulated by interactions between the mRNA and RNA-binding proteins, forming molecular machines known as RNA granules. Various types of these membrane-less organelles form inside cells, including neurons, and contribute critically to various physiological processes. RNA granules are constantly in flux, change dynamically and adapt to their local environment, depending on their intracellular localization. The discovery that RNA condensates can form by liquid–liquid phase separation expanded our understanding of how compartments may be generated in the cell. Since then, a plethora of new functions have been proposed for distinct condensates in cells that await their validation in vivo. The finding that dysregulation of RNA granules (for example, stress granules) is likely to affect neurodevelopmental and neurodegenerative diseases further boosted interest in this topic. RNA granules have various physiological functions in neurons and in the brain that we would like to focus on. We outline examples of state-of-the-art experiments including timelapse microscopy in neurons to unravel the precise functions of various types of RNA granule. Finally, we distinguish physiologically occurring RNA condensation from aberrant aggregation, induced by artificial RNA overexpression, and present visual examples to discriminate both forms in neurons. The physiological dynamics of the molecular machines known as RNA granules have broad implications for neuronal function. In this Review, Kiebler and Bauer discuss the many open questions remaining and highlight recent research, experimental caveats and novel approaches.
变化中的 RNA 颗粒:平衡生理和病理的动态变化
mRNA 的生命周期是一个复杂的过程,受 mRNA 与 RNA 结合蛋白之间相互作用的严格调控,形成称为 RNA 颗粒的分子机器。这些无膜细胞器在包括神经元在内的细胞内形成各种类型,对各种生理过程起着至关重要的作用。RNA 颗粒不断变化,根据其在细胞内的定位,动态地改变和适应局部环境。RNA 凝聚物可以通过液-液相分离形成,这一发现拓展了我们对细胞内如何产生区室的认识。从那时起,人们为细胞中不同的凝聚体提出了大量新功能,这些功能有待在体内验证。研究发现,RNA颗粒(如应激颗粒)的失调可能会影响神经发育和神经退行性疾病,这进一步激发了人们对这一课题的兴趣。RNA 颗粒在神经元和大脑中具有各种生理功能,我们希望重点关注这些功能。我们概述了最先进的实验实例,包括神经元中的延时显微镜,以揭示各类 RNA 颗粒的精确功能。最后,我们将生理上发生的 RNA 凝聚与人工 RNA 过表达诱导的异常聚集区分开来,并通过直观的例子来区分神经元中的这两种形式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Neuroscience
NEUROSCIENCES-
自引率
0.60%
发文量
104
期刊介绍:
Nature Reviews Neuroscience is a multidisciplinary journal that covers various fields within neuroscience, aiming to offer a comprehensive understanding of the structure and function of the central nervous system. Advances in molecular, developmental, and cognitive neuroscience, facilitated by powerful experimental techniques and theoretical approaches, have made enduring neurobiological questions more accessible. Nature Reviews Neuroscience serves as a reliable and accessible resource, addressing the breadth and depth of modern neuroscience. It acts as an authoritative and engaging reference for scientists interested in all aspects of neuroscience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


