Structure, antioxidant activity, and neuroprotective effect of black soybean (Glycine max (L.) merr.) protein hydrolysates
IF 8.5
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The potential biological properties of protein hydrolysates have generated considerable research interest. This study was to hydrolyze black soybean protein (BSP) using five different commercial enzymes, and elucidate the influence of these enzymes on the structure and biological activities of the resulting hydrolysates. Enzymatic treatment changed secondary and tertiary structures of BSP, decreased particle size, α-helix and β-sheet. Alcalase hydrolysate had the highest hydrolytic degree (29.84 %), absolute zeta potential (38.43 mV), the smallest particle (149.87 nm) and molecular weight (<3 kDa). In silico revealed alcalase hydrolysate had the strongest antioxidant potential. This finding was further validated through the lowest IC50 (mg/mL) in DPPH (2.67), ABTS (0.82), Fe2+ chelating (1.33) and·OH (1.12). Moreover, cellular antioxidant assays showed alcalase hydrolysate had the strongest cytoprotective effects on H2O2-induced PC12 cells. These results suggest BSPEHs, especially those prepared by alcalase, have potential as bioactive ingredients for nutrition, healthcare and food industry.
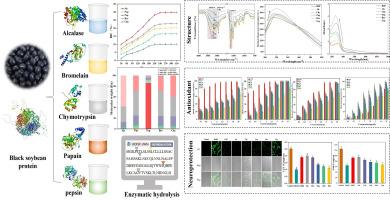
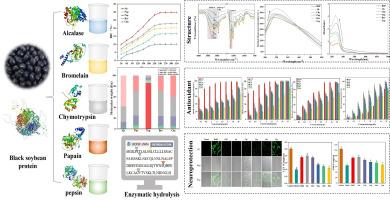
黑大豆(Glycine max (L.) merr.)蛋白水解物的结构、抗氧化活性和神经保护作用
蛋白质水解物的潜在生物特性引起了相当大的研究兴趣。本研究使用五种不同的商业酶水解黑大豆蛋白(BSP),并阐明这些酶对水解产物的结构和生物活性的影响。酶处理改变了黑大豆蛋白的二级和三级结构,减小了粒径、α-螺旋和β-片状结构。醛酶水解物的水解度(29.84 %)、绝对 ZETA 电位(38.43 mV)、最小颗粒(149.87 nm)和分子量(3 kDa)最高。硅学研究表明,脂肪酶水解物具有最强的抗氧化潜力。DPPH (2.67)、ABTS (0.82)、Fe2+ 螯合 (1.33) 和 OH (1.12) 的最低 IC50 (mg/mL) 值进一步验证了这一发现。此外,细胞抗氧化试验表明,丙烯醛酶水解物对 H2O2 诱导的 PC12 细胞具有最强的细胞保护作用。这些结果表明,BSPEHs,尤其是用丙烯醛酶制备的 BSPEHs,具有作为生物活性成分用于营养、保健和食品工业的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
文献相关原料
公司名称
产品信息
上海源叶
ABTS+
上海源叶
2,2-Diphenyl-1-picrylhydrazyl
上海源叶
chymotrypsin
上海源叶
Pepsin
上海源叶
papain
上海源叶
bromelain
上海源叶
Alcalase
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


