Fructose shields human colorectal cancer cells from hypoxia-induced necroptosis
IF 6.3
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
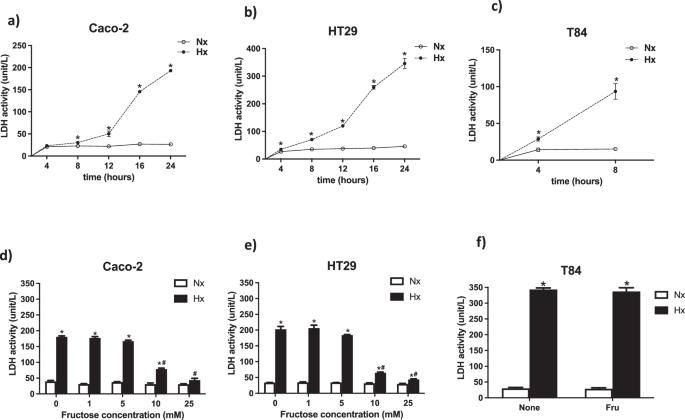
Recent studies have shown that high dietary fructose intake enhances intestinal tumor growth in mice. Our previous work indicated that glucose enables hypoxic colorectal cancer (CRC) cells to resist receptor-interacting protein (RIP)-dependent necroptosis. Despite having the same chemical formula, glucose and fructose are absorbed through different transporters yet both can enter the glycolytic metabolic pathway. The excessive intake of dietary fructose, leading to its overflow into the colon, allows colonic cells to absorb fructose apically. This study explores the mechanisms behind apical fructose-mediated death resistance in CRC cells under hypoxic stress. Utilizing three CRC cell lines (Caco-2, HT29, and T84) under normoxic and hypoxic conditions with varying fructose concentrations, we assessed lactate dehydrogenase (LDH) activity, RIP1/3 complex formation (a necroptosis marker), and cell integrity. We investigated the role of fructose in glycolytic-mediated death resistance using glycolytic inhibitors iodoacetate (IA, a glycolytic inhibitor to glyceraldehyde 3-phosphate dehydrogenase), and UK5099 (UK, an inhibitor to mitochondrial pyruvate carrier). Our findings reveal that apical fructose prevents the hypoxia-induced RIP-dependent necroptosis in Caco-2 and HT29 cells. Fructose exposure under hypoxia also preserved epithelial integrity. IA, but not UK, blocked fructose-mediated glycolytic metabolite production and necrosis, indicating that anaerobic glycolytic metabolites facilitate death resistance. Notably, fructose treatment upregulated pyruvate kinase (PK)-M1 mRNA in hypoxic Caco-2 and HT29 cells, while PKM2 upregulation was exclusive to HT29 cells. In conclusion, apical fructose utilization through glycolysis effectively inhibits hypoxia-induced RIP-dependent necroptosis in CRC cells, shedding light on potential metabolic adaptation mechanisms in the tumor microenvironment and suggesting novel targets for therapeutic intervention.
果糖保护人类结直肠癌细胞免受缺氧诱导的坏死影响
最近的研究表明,摄入大量果糖会促进小鼠肠道肿瘤的生长。我们之前的研究表明,葡萄糖能使缺氧的结直肠癌(CRC)细胞抵抗受体相互作用蛋白(RIP)依赖性坏死。尽管葡萄糖和果糖的化学式相同,但它们通过不同的转运体被吸收,但都能进入糖酵解代谢途径。饮食中摄入过量果糖会导致果糖溢出结肠,从而使结肠细胞从顶部吸收果糖。本研究探讨了在缺氧压力下,果糖介导的 CRC 细胞顶端死亡抵抗背后的机制。我们利用三种 CRC 细胞系(Caco-2、HT29 和 T84),在不同果糖浓度的常氧和缺氧条件下,评估了乳酸脱氢酶(LDH)活性、RIP1/3 复合物形成(一种坏死标志物)和细胞完整性。我们使用糖酵解抑制剂碘乙酸盐(IA,一种3-磷酸甘油醛脱氢酶的糖酵解抑制剂)和UK5099(UK,一种线粒体丙酮酸载体的抑制剂)研究了果糖在糖酵解介导的死亡抵抗中的作用。我们的研究结果表明,在 Caco-2 和 HT29 细胞中,顶端果糖可防止缺氧诱导的 RIP 依赖性坏死。在缺氧条件下接触果糖还能保持上皮细胞的完整性。IA 而不是 UK 能阻止果糖介导的糖酵解代谢产物的产生和坏死,这表明无氧糖酵解代谢产物能促进死亡抵抗。值得注意的是,果糖处理会上调缺氧的 Caco-2 和 HT29 细胞中丙酮酸激酶(PK)-M1 mRNA,而 PKM2 的上调只发生在 HT29 细胞中。总之,通过糖酵解利用顶端果糖能有效抑制缺氧诱导的 RIP 依赖性 CRC 细胞坏死,从而揭示了肿瘤微环境中潜在的代谢适应机制,并提出了新的治疗干预靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Science of Food
FOOD SCIENCE & TECHNOLOGY-
CiteScore
7.50
自引率
1.60%
发文量
53
期刊介绍:
npj Science of Food is an online-only and open access journal publishes high-quality, high-impact papers related to food safety, security, integrated production, processing and packaging, the changes and interactions of food components, and the influence on health and wellness properties of food. The journal will support fundamental studies that advance the science of food beyond the classic focus on processing, thereby addressing basic inquiries around food from the public and industry. It will also support research that might result in innovation of technologies and products that are public-friendly while promoting the United Nations sustainable development goals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


