Cross-species single-cell spatial transcriptomic atlases of the cerebellar cortex
IF 44.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
The molecular and cellular organization of the primate cerebellum remains poorly characterized. We obtained single-cell spatial transcriptomic atlases of macaque, marmoset, and mouse cerebella and identified primate-specific cell subtypes, including Purkinje cells and molecular-layer interneurons, that show different expression of the glutamate ionotropic receptor Delta type subunit 2 (GRID2) gene. Distinct gene expression profiles were found in anterior, posterior, and vestibular regions in all species, whereas region-selective gene expression was predominantly observed in the granular layer of primates and in the Purkinje layer of mice. Gene expression gradients in the cerebellar cortex matched well with functional connectivity gradients revealed with awake functional magnetic resonance imaging, with more lobule-specific differences between primates and mice than between two primate species. These comprehensive atlases and comparative analyses provide the basis for understanding cerebellar evolution and function.
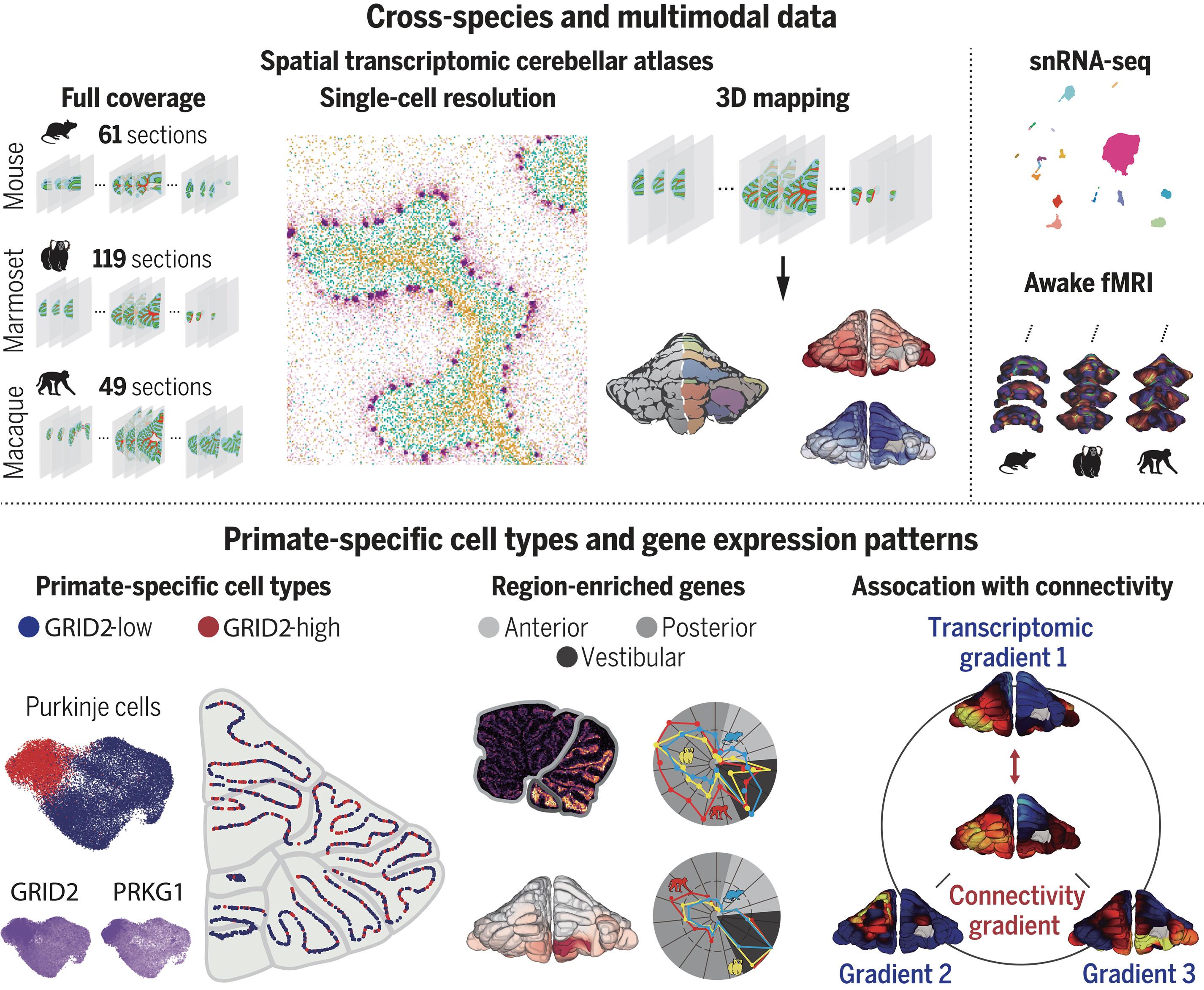
小脑皮层的跨物种单细胞空间转录组图谱
灵长类动物小脑的分子和细胞组织仍然特征不清。我们获得了猕猴、狨猴和小鼠小脑的单细胞空间转录组图谱,并确定了灵长类特有的细胞亚型,包括普肯耶细胞和分子层中间神经元,这些细胞亚型的谷氨酸离子型受体Delta型亚基2(GRID2)基因表现出不同的表达。在所有物种的前庭、后庭和前庭区域都发现了不同的基因表达谱,而在灵长类动物的颗粒层和小鼠的浦肯野层主要观察到了区域选择性基因表达。小脑皮层的基因表达梯度与清醒功能磁共振成像显示的功能连接梯度非常吻合,灵长类动物和小鼠之间的小叶特异性差异比两种灵长类动物之间的差异更大。这些全面的图谱和比较分析为了解小脑的进化和功能奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


