Enzyme Dynamics Determine the Potency and Selectivity of Inhibitors Targeting Disease-Transmitting Mosquitoes
IF 3.8
2区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
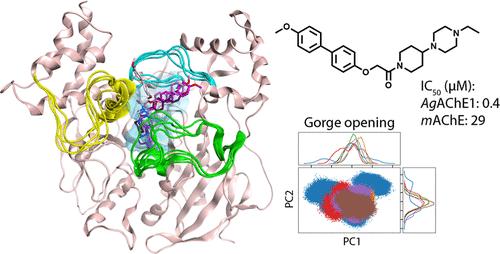
Vector control of mosquitoes with insecticides is an important tool for preventing the spread of mosquito-borne diseases including malaria, dengue, chikungunya, and Zika. Development of active ingredients for insecticides are urgently needed because existing agents exhibit off-target toxicity and are subject to increasing resistance. We therefore seek to develop noncovalent inhibitors of the validated insecticidal target acetylcholinesterase 1 (AChE1) from mosquitoes. Here we use molecular dynamics simulations to identify structural properties essential for the potency of reversible inhibitors targeting AChE1 from Anopheles gambiae (AgAChE1), the malaria-transmitting mosquito, and for selectivity relative to the vertebrate Mus musculus AChE (mAChE). We show that the collective motions of apo AgAChE1 and mAChE differ, with AgAChE1 exhibiting less dynamic movement. Opening and closing of the gorge, which regulates access to the catalytic triad, is enabled by different mechanisms in the two species, which could be linked to their differing amino acid sequences. Inhibitor binding reduced the overall magnitude of dynamics of AChE. In particular, more potent inhibitors reduced the flexibility of the Ω loop at the entrance of the gorge. The selectivity of inhibitors for AgAChE1 over mAChE derives from the positioning of the α-helix lining the binding gorge. Our findings emphasize the need to consider dynamics when developing inhibitors targeting this enzyme and highlight factors needed to create potent and selective AgAChE1 inhibitors that could serve as active ingredients to combat disease-transmitting mosquitoes.
酶动力学决定针对疾病传播蚊虫的抑制剂的效力和选择性
用杀虫剂控制蚊虫病媒是防止疟疾、登革热、基孔肯雅病和寨卡病毒等蚊媒疾病传播的重要手段。由于现有杀虫剂具有脱靶毒性,而且抗药性不断增加,因此迫切需要开发杀虫剂的活性成分。因此,我们试图开发蚊子有效杀虫靶标乙酰胆碱酯酶 1(AChE1)的非共价抑制剂。在这里,我们利用分子动力学模拟来确定针对传播疟疾的冈比亚按蚊(AgAChE1)乙酰胆碱酯酶 1 的可逆抑制剂的效力以及相对于脊椎动物肌肉乙酰胆碱酯酶(mAChE)的选择性所必需的结构特性。我们发现,apo AgAChE1 和 mAChE 的集体运动有所不同,AgAChE1 的动态运动较少。在这两个物种中,调节催化三元组通路的峡谷的打开和关闭是通过不同的机制实现的,这可能与它们不同的氨基酸序列有关。抑制剂的结合降低了 AChE 的整体动态幅度。特别是,更强的抑制剂降低了峡谷入口处Ω环的灵活性。抑制剂对 AgAChE1 而不是 mAChE 的选择性来自于结合峡口α螺旋的位置。我们的发现强调了在开发针对这种酶的抑制剂时考虑动力学因素的必要性,并突出了开发强效和选择性 AgAChE1 抑制剂所需的因素,这些抑制剂可作为抗击传播疾病的蚊子的活性成分。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Infectious Diseases
CHEMISTRY, MEDICINALINFECTIOUS DISEASES&nb-INFECTIOUS DISEASES
CiteScore
9.70
自引率
3.80%
发文量
213
期刊介绍:
ACS Infectious Diseases will be the first journal to highlight chemistry and its role in this multidisciplinary and collaborative research area. The journal will cover a diverse array of topics including, but not limited to:
* Discovery and development of new antimicrobial agents — identified through target- or phenotypic-based approaches as well as compounds that induce synergy with antimicrobials.
* Characterization and validation of drug target or pathways — use of single target and genome-wide knockdown and knockouts, biochemical studies, structural biology, new technologies to facilitate characterization and prioritization of potential drug targets.
* Mechanism of drug resistance — fundamental research that advances our understanding of resistance; strategies to prevent resistance.
* Mechanisms of action — use of genetic, metabolomic, and activity- and affinity-based protein profiling to elucidate the mechanism of action of clinical and experimental antimicrobial agents.
* Host-pathogen interactions — tools for studying host-pathogen interactions, cellular biochemistry of hosts and pathogens, and molecular interactions of pathogens with host microbiota.
* Small molecule vaccine adjuvants for infectious disease.
* Viral and bacterial biochemistry and molecular biology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


