Discovery, Biosynthesis, and Characterization of Rodencin, a Two-Component Lanthipeptide, Harboring d-Amino Acids Introduced by the Unusual Dehydrogenase RodJA
IF 3.3
2区 生物学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
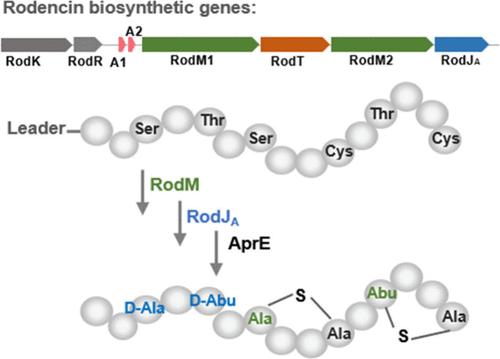
Lanthipeptides, a group of ribosomally synthesized and post-translationally modified peptides (RiPPs), exhibit diverse structures and bioactivities. Their biosynthetic enzymes serve as valuable tools for peptide bioengineering. Here, we report a class II lanthipeptide biosynthetic gene cluster in a Bacillus strain, driving the biosynthesis of a two-component lanthipeptide, termed rodencin, featured by the presence of two different d-amino acids, i.e., d-Ala and d-Abu. Rodencin displays synergistic antimicrobial activity against food-borne pathogens such as Bacillus cereus, Staphylococcus aureus, and Listeria monocytogenes. The α-peptide of rodencin contains one d-Ala and the β-peptide features both d-Ala and d-Abu. These are installed by dehydratases RodM1 and RodM2 and dehydrogenase RodJA, the activities of which were successfully reconstituted using a dedicated E. coli expression system. To illustrate the unusual d-Abu incorporation potential of the enzymes, analogous to the d-amino acid-containing β peptide of lacticin 3147, was successfully produced with the rodencin heterologous expression system, by employing RodM2 and the dehydrogenase RodJA.
发现、生物合成和表征含有由非同寻常的脱氢酶 RodJA 引入的 d-Aminoids 的双组分肽--Rodencin
多肽(Lanthipeptides)是一组核糖体合成和翻译后修饰的多肽(RiPPs),具有多种结构和生物活性。它们的生物合成酶是肽生物工程的重要工具。在这里,我们报告了一个芽孢杆菌菌株中的 II 类兰肽生物合成基因簇,该基因簇驱动了一种双组分兰肽的生物合成,这种兰肽被称为 Rodencin,其特点是含有两种不同的 d-氨基酸,即 d-Ala 和 d-Abu。棒曲霉素对蜡样芽孢杆菌、金黄色葡萄球菌和李斯特菌等食源性病原体具有协同抗菌活性。鼠得克肽的 α 肽含有一个 d-Ala,而 β 肽含有 d-Ala 和 d-Abu。这些物质由脱水酶 RodM1 和 RodM2 以及脱氢酶 RodJA 安装,其活性已通过专用的大肠杆菌表达系统成功重组。为了说明酶的不寻常的 d-Abu 结合潜力,利用 RodM2 和脱氢酶 RodJA,成功地在棒曲霉素异源表达系统中生产出类似于乳酸素 3147 的含 d- 氨基酸的 β 肽。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


