The Antiphytopathogenic Metabolites and the Synergy of Endophytes Paraphaeosphaeria sp., Nigrospora oryzae from the Same Ginkgo biloba Host
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Two endophytes from the same Ginkgo biloba host were isolated and cultured separately. Three new eremophilane sesquiterpenoids (1–3), three new furan derivates (6, 8–9), one new polyketide (10), and four known compounds (4, 5, 7, 11) from Paraphaeosphaeria sp. and two new 10-membered macrolides (12–13), a new liner polyketide (14), a new benzofuran (15), and six known compounds (16–21) from Nigrospora oryzae were isolated. The structures of the isolated compounds were determined by spectroscopic methods, NMR calculations, and ECD calculations. The compounds 3–7, 9–10, 12, and 14–17 showed significant antiphytopathogenic effects against mycotoxigenic Alternaria sp. comparable to the activity of nystatin (positive control). Compounds 2, 6, 8, 9, and 18 indicated inhibitions against phytopathogen Fusarium asiaticum with MICs < 10 μg/mL. In addition, the compounds with weak antifungal activities from two endophytes were mixed to test their antifungal activity. The results showed that the metabolites from two endophytes had synergistic antifungal effects, and the beneficial interactions between natural products can induce more antifungal effects against plant pathogens than that of single compounds.
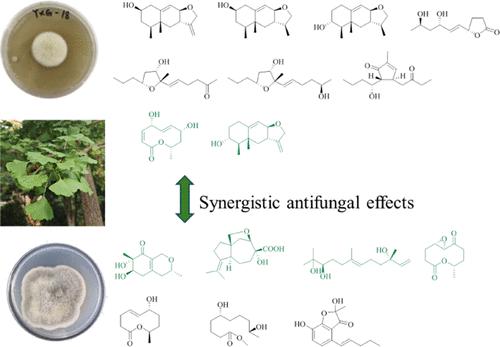
来自同一银杏寄主的内生菌 Paraphaeosphaeria sp.
从同一银杏寄主中分离并分别培养了两种内生菌。从 Paraphaeosphaeria sp.中分离出了 3 个新的 eremophilane 倍半萜类化合物(1-3)、3 个新的呋喃衍生物(6, 8-9)、1 个新的多酮类化合物(10)和 4 个已知化合物(4, 5, 7, 11);从 Nigrospora oryzae 中分离出了 2 个新的 10 元大环内酯类化合物(12-13)、1 个新的衬多酮类化合物(14)、1 个新的苯并呋喃类化合物(15)和 6 个已知化合物(16-21)。分离出的化合物的结构是通过光谱方法、核磁共振计算和 ECD 计算确定的。化合物 3-7、9-10、12 和 14-17 对霉菌毒素 Alternaria sp.有显著的抗致病作用,其活性与硝司他丁(阳性对照)相当。化合物 2、6、8、9 和 18 对植物病原镰刀菌有抑制作用,其 MICs 为 10 μg/mL。此外,还混合了两种内生菌中抗真菌活性较弱的化合物,以测试它们的抗真菌活性。结果表明,两种内生菌的代谢产物具有协同抗真菌作用,天然产物之间的有益相互作用比单一化合物对植物病原体的抗真菌作用更强。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


