Target Fishing Reveals a Novel Mechanism of N-Acylamino Saccharin Derivatives Targeting Glyceraldehyde-3-Phosphate Dehydrogenase toward Cyanobacterial Blooms Control
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Based on current challenges of poor targeting and limited choices in chemical control methods of cyanobacterial blooms (CBs), identifying new targets is an urgent and formidable task in the quest for target-based algaecides. This study discovered N-acylamino saccharin derivatives exhibiting potent algicidal activity. Thus, using N-acylamino saccharin as the probes, glyceraldehyde-3-phosphate dehydrogenase from cyanobacterial (CyGAPDH) was identified as a new target of algaecides through the activity-based protein profiling (ABPP) strategy for the first time. Building upon the structure of Probe2, a series of derivatives were designed and synthesized, with compound b6 demonstrating the most potent inhibitory activity against CyGAPDH and Synechocystis sp. PCC6803 (IC50 = 1.67 μM and EC50 = 1.15 μM). Furthermore, the potential covalent binding model of b6 to the cysteine residue C154 was explored through covalent possibility prediction, LC–MS experiments, substrate competitive inhibition experiments, and molecular docking. Especially, the results revealed C154 as a crucial covalent binding site, with residues T184 and R11 forming robust hydrophobic interactions and H181 establishing significant hydrogen-bonding interactions with b6, highlighting their potential as essential pharmacophores. In summary, this study not only identifies a novel target of algaecides for the control of CB but also lays the solid foundation for the development of targeted covalent algaecides.
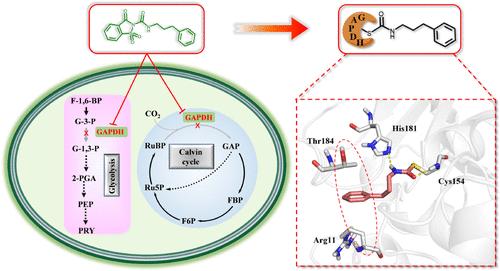
靶标捕捉揭示了 N-酰氨基糖精衍生物靶向甘油醛-3-磷酸脱氢酶控制蓝藻藻华的新机制
鉴于目前蓝藻水华(CBs)化学防治方法存在靶向性差和选择有限的挑战,确定新的靶标是寻找基于靶标的杀藻剂的一项紧迫而艰巨的任务。本研究发现了具有强力杀藻活性的 N-酰氨基糖精衍生物。因此,以 N-酰氨基糖精为探针,通过基于活性的蛋白质分析(ABPP)策略,首次发现了蓝藻中的甘油醛-3-磷酸脱氢酶(CyGAPDH)作为杀藻剂的新靶标。根据 Probe2 的结构,设计并合成了一系列衍生物,其中化合物 b6 对 CyGAPDH 和 Synechocystis sp.此外,还通过共价可能性预测、LC-MS 实验、底物竞争抑制实验和分子对接等方法探讨了 b6 与半胱氨酸残基 C154 的潜在共价结合模型。研究结果表明,C154 是关键的共价结合位点,残基 T184 和 R11 与 b6 形成了强大的疏水相互作用,H181 与 b6 建立了显著的氢键相互作用,凸显了它们作为重要药效物质的潜力。总之,这项研究不仅确定了杀藻剂控制 CB 的新靶标,还为开发有针对性的共价杀藻剂奠定了坚实的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


