Electrophoresis-Correlative Data-Independent Acquisition (Eco-DIA) Improves the Sensitivity of Mass Spectrometry for Limited Proteome Amounts
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Capillary zone electrophoresis (CE) combines high separation power, scalability, and speed to limited proteome analyses by mass spectrometry (MS). However, compressed separation in CE challenges the duty cycle of tandem MS, even during data-independent acquisition (DIA). To help remedy this limitation, we introduce the concept of electrophoresis-correlative (Eco) data acquisition for CE-MS. We recognize CE electrospray ionization (ESI) to sort peptide ions into reproducible mass-to-charge (m/z) vs migration time (MT) trends in the solution phase, before subsequent ionization and m/z analysis. We proposed that such a correlation can be leveraged to improve the economy of data acquisition. We test this hypothesis using DIA frames that are tailored to the observed m/z–MT trends. The resulting Eco-DIA method substantially improves the bandwidth utilization of tandem MS during CE-MS. In proof-of-principle studies, Eco-DIA identified and quantified ∼38% more proteins from 1 ng of the HeLa proteome digest compared to the classical DIA, without the assistance of a project-specific tandem MS spectral library. Eco-DIA was able to quantify ∼51% more proteins with <10% coefficient of variation vs the control DIA approach. Based on label-free quantification, the proteins that were exclusively measured by Eco-MS occupied the lower dynamic range of the detected proteome concentration, revealing sensitivity enhancement. In addition to marking the inception of Eco-MS, this work lays the foundation for the development of next-generation data acquisition strategies that leverage electrophoretic ion sorting for high-sensitivity proteomics.
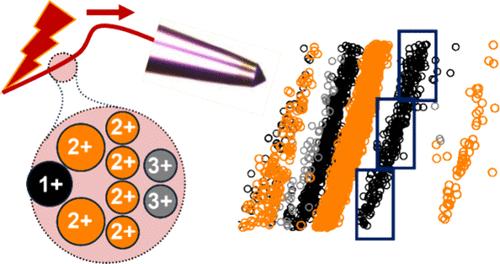
电泳-相关数据独立采集(Eco-DIA)提高了质谱法对有限蛋白质组的灵敏度
毛细管区带电泳(CE)集高分离能力、可扩展性和速度于一身,限制了质谱(MS)对蛋白质组的分析。然而,毛细管区电泳中的压缩分离对串联质谱的占空比提出了挑战,即使在独立数据采集(DIA)期间也是如此。为了弥补这一不足,我们为 CE-MS 引入了电泳相关(Eco)数据采集的概念。我们认识到 CE 电喷雾离子化(ESI)可在溶液阶段将肽离子按可重现的质量-电荷(m/z)与迁移时间(MT)趋势分类,然后再进行离子化和 m/z 分析。我们建议利用这种相关性来提高数据采集的经济性。我们使用根据观察到的 m/z-MT 趋势定制的 DIA 框架来验证这一假设。由此产生的 Eco-DIA 方法大大提高了 CE-MS 期间串联质谱的带宽利用率。在原理验证研究中,与传统的 DIA 相比,Eco-DIA 从 1 纳克 HeLa 蛋白质组消化物中鉴定和量化的蛋白质数量增加了 38%,而且无需特定项目的串联质谱库。与对照 DIA 方法相比,Eco-DIA 定量的蛋白质数量增加了 51%,变异系数为 10%。在无标记定量的基础上,完全由 Eco-MS 测定的蛋白质占据了检测到的蛋白质组浓度的较低动态范围,显示了灵敏度的提高。除了标志着 Eco-MS 的诞生,这项研究还为开发下一代数据采集策略奠定了基础,这些策略可利用电泳离子分选技术实现高灵敏度蛋白质组学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


