Benzobisthiazole unit in 4,8-connection mode to build D–A polymer donors achieving high short-circuit current density for organic solar cells
IF 5.7
2区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
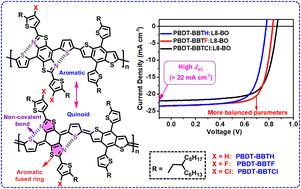
In this work, a series of D–A conjugated polymer donors (namely PBDT-BBTH, PBDT-BBTF and PBDT-BBTCl) was designed based on the benzobisthiazole (BBT) unit in the 4,8-connection mode with the benzodithiophene (BDT) unit linked by the thiophene π-bridge. At the same time, an α-alkyl-thiophene ring with different β-atoms (H, F and Cl) was introduced at 2,6-positions of the BBT unit as a side-chain to extend the conjugation and regulate the energy levels. In 4,8-connection mode, the aromatic fused-thiazole ring of the BBT unit can stabilize the quinoid configuration of the main chain to strengthen the intramolecular charge transfer (ICT) and improve the π-electron delocalization of the conjugated backbone. The DFT calculations indicate that there exists the N⋯S noncovalent interaction between the BBT unit and the adjacent thiophene π-bridge that can lock the main-chain conformation to enhance the rigidity of the conjugated backbone. Thus, these polymers exhibit strong absorption in the range of 300–650 nm, which is favorable for light-harvesting to improve the short-circuit current density (JSC) of the organic solar cells (OSCs). In addition, the introduction of a strongly electronegative F or Cl atom at the β-position of the α-alkyl-thiophene side-chain can reduce the HOMO energy level, which is beneficial for the enhancement of the open-circuit voltage (VOC). Finally, the optimized OSC devices based on these polymers with an L8-BO acceptor exhibit good JSC over 22 mA cm−2, and the devices based on PBDT-BBTF and PBDT-BBTCl show higher VOC than the device based on PBDT-BBTH. Among these polymers, the PBDT-BBTF-based device achieves more balanced parameters (JSC = 23.43 mA cm−2, VOC = 0.831 V, and FF = 73.36%) to lead to the best PCE of 14.27%.
以 4,8 连接模式构建 D-A 型聚合物供体的苯并二噻唑单元,实现有机太阳能电池的高短路电流密度
在这项工作中,我们设计了一系列 D-A 共轭聚合物给体(即 PBDT-BBTH、PBDT-BBTF 和 PBDT-BBTCl),它们基于 4,8 连接模式的苯并二噻唑(BBT)单元和通过噻吩 π 桥连接的苯并二噻吩(BDT)单元。同时,在 BBT 单元的 2,6 位引入了一个具有不同 β 原子(H、F 和 Cl)的 α 烷基噻吩环作为侧链,以扩展共轭作用并调节能级。在 4,8 连接模式下,BBT 单元的芳香熔合噻唑环可以稳定主链的醌构型,从而加强分子内电荷转移(ICT),并改善共轭骨架的 π 电子分散。DFT 计算表明,BBT 单元与相邻的噻吩 π 桥之间存在 N⋯S 非共价相互作用,可以锁定主链构象,增强共轭骨架的刚性。因此,这些聚合物在 300-650 纳米范围内具有很强的吸收能力,有利于光收集,从而提高有机太阳能电池(OSC)的短路电流密度(JSC)。此外,在α-烷基噻吩侧链的β位引入强电负性的F或Cl原子可以降低HOMO能级,有利于提高开路电压(VOC)。最后,基于这些聚合物和 L8-BO 受体的优化 OSC 器件显示出良好的 JSC,超过 22 mA cm-2,基于 PBDT-BBTF 和 PBDT-BBTCl 的器件比基于 PBDT-BBTH 的器件显示出更高的 VOC。在这些聚合物中,基于 PBDT-BBTF 的器件获得了更均衡的参数(JSC = 23.43 mA cm-2、VOC = 0.831 V 和 FF = 73.36%),从而实现了 14.27% 的最佳 PCE。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Chemistry C
MATERIALS SCIENCE, MULTIDISCIPLINARY-PHYSICS, APPLIED
CiteScore
10.80
自引率
6.20%
发文量
1468
期刊介绍:
The Journal of Materials Chemistry is divided into three distinct sections, A, B, and C, each catering to specific applications of the materials under study:
Journal of Materials Chemistry A focuses primarily on materials intended for applications in energy and sustainability.
Journal of Materials Chemistry B specializes in materials designed for applications in biology and medicine.
Journal of Materials Chemistry C is dedicated to materials suitable for applications in optical, magnetic, and electronic devices.
Example topic areas within the scope of Journal of Materials Chemistry C are listed below. This list is neither exhaustive nor exclusive.
Bioelectronics
Conductors
Detectors
Dielectrics
Displays
Ferroelectrics
Lasers
LEDs
Lighting
Liquid crystals
Memory
Metamaterials
Multiferroics
Photonics
Photovoltaics
Semiconductors
Sensors
Single molecule conductors
Spintronics
Superconductors
Thermoelectrics
Topological insulators
Transistors
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


