Identification of Chemical Scaffolds That Inhibit the Mycobacterium tuberculosis Respiratory Complex Succinate Dehydrogenase
IF 4
2区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
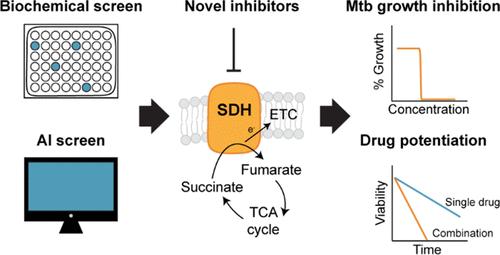
Drug-resistant Mycobacterium tuberculosis is a significant cause of infectious disease morbidity and mortality for which new antimicrobials are urgently needed. Inhibitors of mycobacterial respiratory energy metabolism have emerged as promising next-generation antimicrobials, but a number of targets remain unexplored. Succinate dehydrogenase (SDH), a focal point in mycobacterial central carbon metabolism and respiratory energy production, is required for growth and survival in M. tuberculosis under a number of conditions, highlighting the potential of inhibitors targeting mycobacterial SDH enzymes. To advance SDH as a novel drug target in M. tuberculosis, we utilized a combination of biochemical screening and in-silico deep learning technologies to identify multiple chemical scaffolds capable of inhibiting mycobacterial SDH activity. Antimicrobial susceptibility assays show that lead inhibitors are bacteriostatic agents with activity against wild-type and drug-resistant strains of M. tuberculosis. Mode of action studies on lead compounds demonstrate that the specific inhibition of SDH activity dysregulates mycobacterial metabolism and respiration and results in the secretion of intracellular succinate. Interaction assays demonstrate that the chemical inhibition of SDH activity potentiates the activity of other bioenergetic inhibitors and prevents the emergence of resistance to a variety of drugs. Overall, this study shows that SDH inhibitors are promising next-generation antimicrobials against M. tuberculosis.
鉴定抑制结核分枝杆菌呼吸复合体琥珀酸脱氢酶的化学支架
耐药性结核分枝杆菌是传染病发病率和死亡率的一个重要原因,因此迫切需要新的抗菌药物。分枝杆菌呼吸能量代谢抑制剂已成为有希望的下一代抗菌药物,但仍有许多靶点尚未开发。琥珀酸脱氢酶(SDH)是分枝杆菌中心碳代谢和呼吸能量产生的一个焦点,结核杆菌在多种条件下的生长和存活都需要SDH,这凸显了靶向分枝杆菌SDH酶的抑制剂的潜力。为了推动 SDH 成为结核杆菌的新型药物靶点,我们结合生化筛选和体内深度学习技术,鉴定了多种能够抑制分枝杆菌 SDH 活性的化学支架。抗菌药敏感性试验表明,先导抑制剂是一种抑菌剂,对野生型和耐药结核杆菌株具有活性。对先导化合物的作用模式研究表明,对 SDH 活性的特异性抑制会使分枝杆菌的新陈代谢和呼吸失调,并导致细胞内琥珀酸的分泌。相互作用试验表明,对 SDH 活性的化学抑制可增强其他生物能抑制剂的活性,并防止出现对多种药物的抗药性。总之,这项研究表明,SDH 抑制剂是很有前途的下一代结核杆菌抗菌剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Infectious Diseases
CHEMISTRY, MEDICINALINFECTIOUS DISEASES&nb-INFECTIOUS DISEASES
CiteScore
9.70
自引率
3.80%
发文量
213
期刊介绍:
ACS Infectious Diseases will be the first journal to highlight chemistry and its role in this multidisciplinary and collaborative research area. The journal will cover a diverse array of topics including, but not limited to:
* Discovery and development of new antimicrobial agents — identified through target- or phenotypic-based approaches as well as compounds that induce synergy with antimicrobials.
* Characterization and validation of drug target or pathways — use of single target and genome-wide knockdown and knockouts, biochemical studies, structural biology, new technologies to facilitate characterization and prioritization of potential drug targets.
* Mechanism of drug resistance — fundamental research that advances our understanding of resistance; strategies to prevent resistance.
* Mechanisms of action — use of genetic, metabolomic, and activity- and affinity-based protein profiling to elucidate the mechanism of action of clinical and experimental antimicrobial agents.
* Host-pathogen interactions — tools for studying host-pathogen interactions, cellular biochemistry of hosts and pathogens, and molecular interactions of pathogens with host microbiota.
* Small molecule vaccine adjuvants for infectious disease.
* Viral and bacterial biochemistry and molecular biology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


