Antimicrobial Dihydroflavonols and Isoflavans Isolated from the Root Bark of Dalbergia gloveri
IF 3.6
2区 生物学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Three new dihydroflavonols, gloverinols A–C (1–3), a new flavon-3-ol, gloverinol D (4), two new isoflavans, gloveriflavan A (5) and B (6), and seven known compounds were isolated from the root bark of Dalbergia gloveri. The structures of the isolates were elucidated by using NMR, ECD, and HRESIMS data analyses. Among the isolated compounds, gloverinol B (2), gloveriflavan B (6), and 1-(2,4-dihydroxyphenyl)-3-hydroxy-3-(4-hydroxyphenyl)-1-propanone (10) were the most active against Staphylococcus aureus, with MIC values of 9.2, 18.4, and 14.2 μM, respectively.
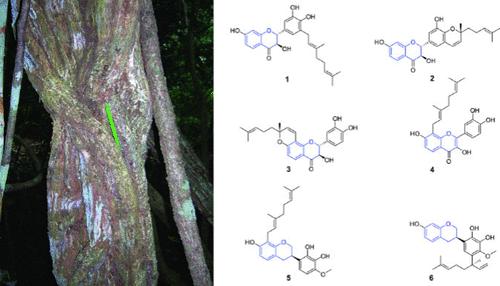
从 Dalbergia gloveri 根皮中分离出的抗菌二氢黄酮醇和异黄酮素
从 Dalbergia gloveri 的根皮中分离出了三种新的二氢黄酮醇,即 gloverinols A-C (1-3),一种新的黄酮-3-醇,即 gloverinol D (4),两种新的异黄酮,即 gloveriflavan A (5) 和 B (6),以及七种已知化合物。通过核磁共振、电离辐射和 HRESIMS 数据分析,阐明了分离物的结构。在分离出的化合物中,gloverinol B(2)、gloveriflavan B(6)和 1-(2,4-二羟基苯基)-3-羟基-3-(4-羟基苯基)-1-丙酮(10)对金黄色葡萄球菌的活性最强,其 MIC 值分别为 9.2、18.4 和 14.2 μM。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


