Parthenolide Inhibits Synthesis and Promotes Degradation of Programmed Cell Death Ligand 1 and Enhances T Cell Tumor-Killing Activity
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Parthenolide is a germacrane sesquiterpene lactone separated from the traditional medicinal plant feverfew. Previous studies have shown that parthenolide possesses many pharmacological activities, involving anti-inflammatory and anticancer activities. However, the antitumor mechanism of parthenolide has not been fully elucidated. Thus, we investigate the potential antitumor mechanisms of parthenolactone. We predicted through network pharmacology that parthenolide may target HIF-1α to interfere with the occurrence and development of cancer. We found that parthenolide inhibited PD-L1 protein synthesis through mTOR/p70S6K/4EBP1/eIF4E and RAS/RAF/MEK/MAPK signaling pathways and promoted PD-L1 protein degradation through the lysosomal pathway, thereby inhibiting PD-L1 expression. Immunoprecipitation and Western blotting results demonstrated that parthenolide inhibited PD-L1 expression by suppressing HIF-1α and RAS cooperatively. We further proved that parthenolide inhibited cell proliferation, migration, invasion, and tube formation via down-regulating PD-L1. Moreover, parthenolide increased the effect of T cells to kill tumor cells. In vivo xenograft assays further demonstrated that parthenolide suppressed the growth of tumor xenografts. Collectively, we report for the first time that parthenolide enhanced T cell tumor-killing activity and suppressed cell proliferation, migration, invasion, and tube formation by PD-L1. The current study provides new insight for the development of parthenolide as a novel anticancer drug targeting PD-L1.
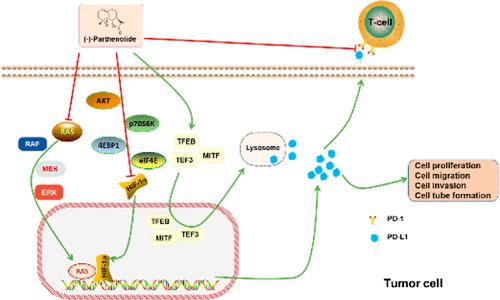
帕芬内酯抑制程序性细胞死亡配体 1 的合成并促进其降解,增强 T 细胞杀伤肿瘤的活性
Parthenolide 是一种从传统药用植物发热草中分离出来的锗萜倍半萜内酯。以往的研究表明,马钱子内酯具有多种药理活性,包括抗炎和抗癌活性。然而,目前还没有完全阐明其抗肿瘤机制。因此,我们对半内酯的潜在抗肿瘤机制进行了研究。我们通过网络药理学预测,非那内酯可能会靶向 HIF-1α,从而干扰癌症的发生和发展。我们发现,parthenolide可通过mTOR/p70S6K/4EBP1/eIF4E和RAS/RAF/MEK/MAPK信号通路抑制PD-L1蛋白合成,并通过溶酶体通路促进PD-L1蛋白降解,从而抑制PD-L1的表达。免疫沉淀和 Western 印迹结果表明,parthenolide 通过协同抑制 HIF-1α 和 RAS 来抑制 PD-L1 的表达。我们进一步证明,非那西丁内酯通过下调 PD-L1 抑制了细胞的增殖、迁移、侵袭和管形成。此外,非那西丁内酯还能增强T细胞杀伤肿瘤细胞的效果。体内异种移植实验进一步证明,parthenolide 能抑制肿瘤异种移植的生长。总之,我们首次报道了非那西丁内酯能增强 T 细胞杀伤肿瘤细胞的活性,并通过 PD-L1 抑制细胞增殖、迁移、侵袭和管形成。目前的研究为开发以 PD-L1 为靶点的新型抗癌药物 parthenolide 提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


