A novel in-silico strategy for the combined inhibition of intestinal bacterial resistance and the transfer of resistant genes using new fluoroquinolones, antibiotic adjuvants, and phytochemicals
Abstract
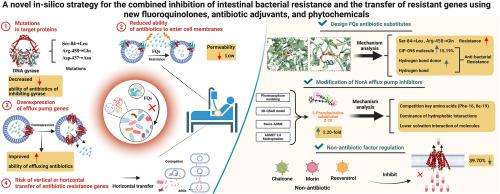
The antibiotic resistance and transfer of antibiotic resistance genes (ARGs) lead to severe environmental threats, and efficient regulatory measures to solve the above problems are urgently needed. Thus, a novel three-dimensional quantitative structure-activity relationship for S. aureus antibiotic resistance was constructed in this study. A fluoroquinolone (FQ) substitute (CIP-098) with decreased bacterial resistance by 15.19% and antibiotic adjuvant (2-phenylquinoline efflux pump inhibitor (2P-Q-EPI) substitute (Z-20)) that enhanced efflux pump inhibition by 1.96 times were designed. Mechanism analysis revealed that hydrogen bond donors and hydrogen bonding in FQ are essential groups and non-covalent interactions, which assist antibiotics in combating resistance mutations in S. aureus's DNA gyrase that transition from hydrophilic to hydrophobic residues. Z-20 was found to easily bind to key amino acid residues (Phe-16, Ile-19), thus reducing the antibiotic expulsion by the NorA efflux pump protein, which can inhibit antibiotic resistance in bacteria. The non-antibiotic factor regulatory scheme designed in this study significantly reduced (by 39.70%) the efflux of FQ by S. aureus and the risk of horizontal ARGs transfer. This study proposes a new strategy to mitigate FQ antibiotic resistance and ARGs transfer in gut microbiota, offering technical support for the green development of FQ antibiotics and 2P-Q-EPI.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


