ImAge quantitates aging and rejuvenation
IF 17
Q1 CELL BIOLOGY
引用次数: 0
Abstract
For efficient, cost-effective and personalized healthcare, biomarkers that capture aspects of functional, biological aging, thus predicting disease risk and lifespan more accurately and reliably than chronological age, are essential. We developed an imaging-based chromatin and epigenetic age (ImAge) that captures intrinsic age-related trajectories of the spatial organization of chromatin and epigenetic marks in single nuclei, in mice. We show that such trajectories readily emerge as principal changes in each individual dataset without regression on chronological age, and that ImAge can be computed using several epigenetic marks and DNA labeling. We find that interventions known to affect biological aging induce corresponding effects on ImAge, including increased ImAge upon chemotherapy treatment and decreased ImAge upon caloric restriction and partial reprogramming by transient OSKM expression in liver and skeletal muscle. Further, ImAge readouts from chronologically identical mice inversely correlated with their locomotor activity, suggesting that ImAge may capture elements of biological and functional age. In sum, we developed ImAge, an imaging-based biomarker of aging with single-cell resolution rooted in the analysis of spatial organization of epigenetic marks. Alvarez-Kuglen, Ninomiya, Qin, Rodriguez et al. demonstrate that the spatial organization of chromatin and epigenetic marks in individual nuclei follows age-related trajectories that can be captured by an imaging-based biomarker of aging (ImAge).
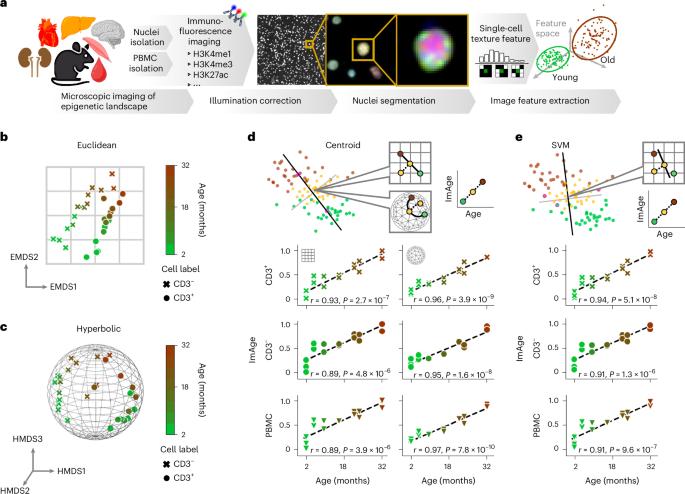
ImAge 对衰老和年轻化进行量化。
为了实现高效、经济和个性化的医疗保健,必须有生物标志物来捕捉功能性生物衰老的各个方面,从而比计时年龄更准确可靠地预测疾病风险和寿命。我们开发了一种基于成像的染色质和表观遗传年龄(ImAge),它能捕捉小鼠单个细胞核中染色质和表观遗传标记的空间组织与年龄相关的内在轨迹。我们的研究表明,这种轨迹很容易在每个数据集中显示为主要变化,而不需要对年代年龄进行回归,而且可以使用几种表观遗传标记和 DNA 标记来计算 ImAge。我们发现,已知会影响生物衰老的干预措施会对 ImAge 产生相应的影响,包括化疗后 ImAge 增加、热量限制后 ImAge 减少,以及在肝脏和骨骼肌中通过瞬时 OSKM 表达进行部分重编程。此外,时间相同的小鼠的 ImAge 读数与它们的运动活动成反比,这表明 ImAge 可能捕捉到生物和功能年龄的要素。总之,我们开发了 ImAge,这是一种基于成像的衰老生物标记,具有单细胞分辨率,根植于表观遗传标记的空间组织分析。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


