Micronuclear collapse from oxidative damage
IF 44.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Chromosome-containing micronuclei are a hallmark of aggressive cancers. Micronuclei frequently undergo irreversible collapse, exposing their enclosed chromatin to the cytosol. Micronuclear rupture catalyzes chromosomal rearrangements, epigenetic abnormalities, and inflammation, yet mechanisms safeguarding micronuclear integrity are poorly understood. In this study, we found that mitochondria-derived reactive oxygen species (ROS) disrupt micronuclei by promoting a noncanonical function of charged multivesicular body protein 7 (CHMP7), a scaffolding protein for the membrane repair complex known as endosomal sorting complex required for transport III (ESCRT-III). ROS retained CHMP7 in micronuclei while disrupting its interaction with other ESCRT-III components. ROS-induced cysteine oxidation stimulated CHMP7 oligomerization and binding to the nuclear membrane protein LEMD2, disrupting micronuclear envelopes. Furthermore, this ROS-CHMP7 pathological axis engendered chromosome shattering known to result from micronuclear rupture. It also mediated micronuclear disintegrity under hypoxic conditions, linking tumor hypoxia with downstream processes driving cancer progression.
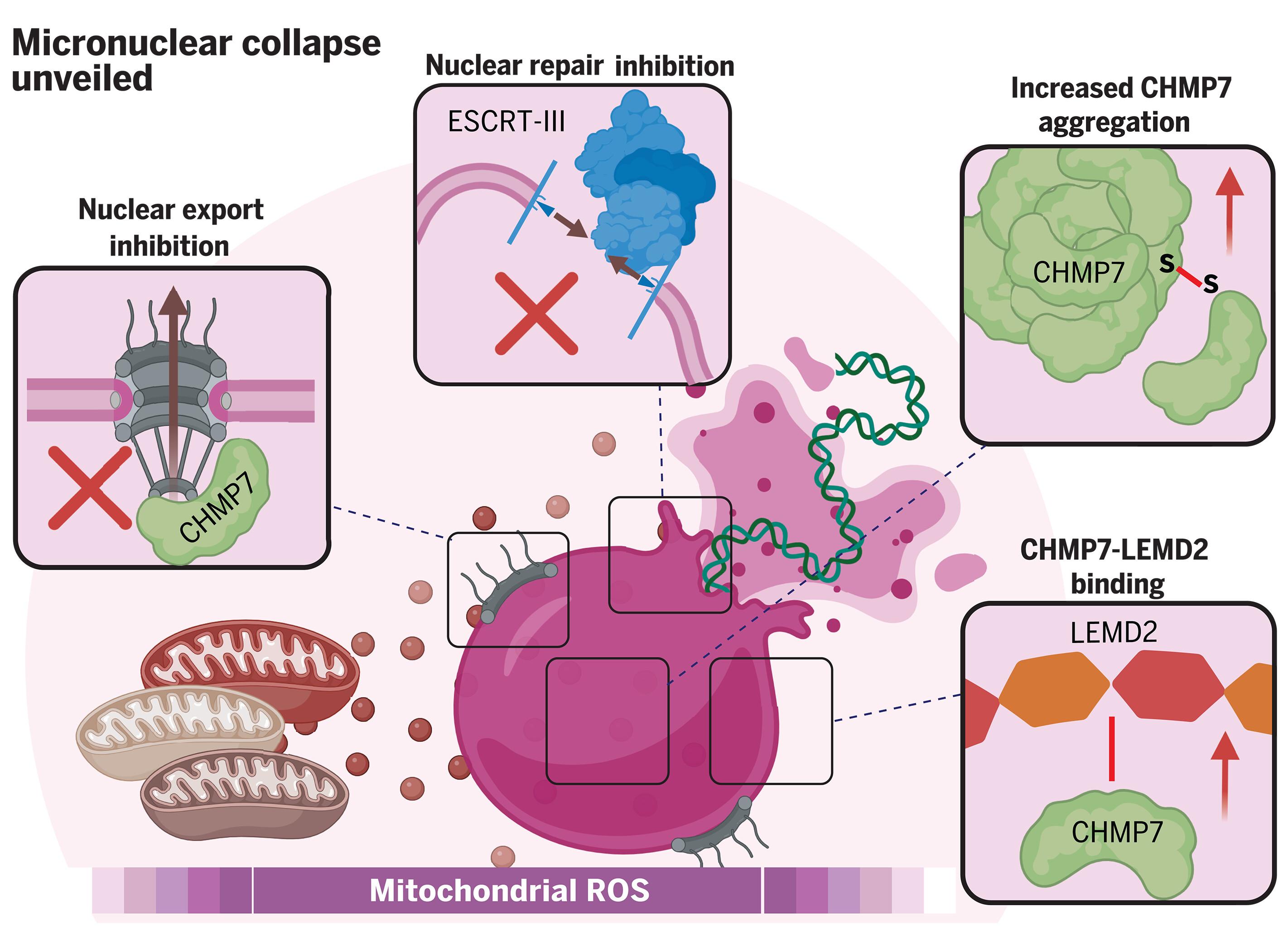
氧化损伤导致的微核崩溃
含染色体的微核是侵袭性癌症的标志。微核经常发生不可逆转的崩解,使其封闭的染色质暴露在细胞质中。微核破裂会催化染色体重排、表观遗传异常和炎症,但人们对保护微核完整性的机制却知之甚少。在这项研究中,我们发现线粒体衍生的活性氧(ROS)通过促进带电多囊体蛋白 7(CHMP7)的非经典功能来破坏微核,而带电多囊体蛋白 7 是膜修复复合物的支架蛋白,被称为转运所需的内体分选复合物 III(ESCRT-III)。ROS 将 CHMP7 保留在微核中,同时破坏了它与其他 ESCRT-III 成分的相互作用。ROS 诱导的半胱氨酸氧化刺激了 CHMP7 的寡聚化并与核膜蛋白 LEMD2 结合,从而破坏了微核包膜。此外,ROS-CHMP7病理轴还会导致染色体破碎,而染色体破碎正是微核破裂的结果。它还在缺氧条件下介导微核解体,将肿瘤缺氧与驱动癌症进展的下游过程联系起来。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


