Endogenous Messenger RNA-Stimulative Self-Enhanced Orthogonal Catalytic DNA Nanomachine under Near-Infrared Light Mediation for High-Performance Imaging in Living Biosamples
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
While the higher assay sensitivity makes orthogonal catalytic DNA nanomachines promising for imaging analysis in living biosamples, the absence of specific recognition components and the persistent “always-on” state for biomolecular identification hinder their further application. Hereby, we establish an endogenous mRNA (mRNA)-stimulative self-enhanced orthogonal catalytic DNA nanomachine mediated by near-infrared (NIR) light. Initially, a catalytic hairpin assembly and entropy-driven catalysis cascaded self-enhanced circuit is equipped with a component specific to TK1 mRNA recognition, enabling highly selective sensing initiation. Furthermore, the interior of a DNA segment is inserted with a photocleavable bond to lock the biomolecular identification, followed by NIR light mediation of up-converting luminescence to avoid premature activation of the sensing system during biodelivery. By selecting a cancer-correlated noncoding microRNA biomarker (miRNA-21) for conceptual illustration, this newly devised DNA nanomachine exhibits remarkably sensitive sensing capability with good specificity in solution detection. Delving deeper, our sensing methodology reliably achieves high-performance imaging of this low-abundance target in living biosamples, covering both the cellular and in vivo levels. This drives forward the advancement of DNA nanomachine-built fluorescent biosensors in disease diagnosis.
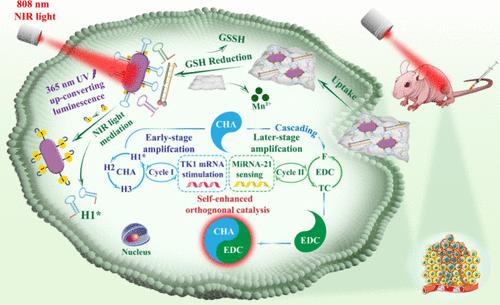
近红外光介导下的内源性信使 RNA-刺激性自增强正交催化 DNA 纳米机器,用于活体生物样本的高性能成像
虽然较高的检测灵敏度使正交催化 DNA 纳米机械有望用于活体生物样本的成像分析,但缺乏特异性识别元件和生物分子识别的持续 "始终开启 "状态阻碍了它们的进一步应用。因此,我们建立了一种由近红外(NIR)光介导的内源性 mRNA(mRNA)刺激性自增强正交催化 DNA 纳米机械。首先,催化发夹组装和熵驱动催化级联自增强电路配备了一个特异性 TK1 mRNA 识别组件,从而实现了高度选择性的感应启动。此外,DNA 片段的内部插入了一个光可裂解键,以锁定生物分子识别,然后用近红外光调解上转换发光,以避免在生物递送过程中过早激活传感系统。通过选择一种与癌症相关的非编码 microRNA 生物标记物(miRNA-21)作为概念性说明,这种新设计的 DNA 纳米机械在溶液检测中表现出非常灵敏的传感能力和良好的特异性。更深入地说,我们的传感方法可靠地实现了这种低丰度目标在活体生物样本中的高性能成像,涵盖了细胞和体内两个层面。这推动了 DNA 纳米机械荧光生物传感器在疾病诊断领域的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


