Multiscale photocatalytic proximity labeling reveals cell surface neighbors on and between cells
IF 44.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Proximity labeling proteomics (PLP) strategies are powerful approaches to yield snapshots of protein neighborhoods. Here, we describe a multiscale PLP method with adjustable resolution that uses a commercially available photocatalyst, Eosin Y, which upon visible light illumination activates different photo-probes with a range of labeling radii. We applied this platform to profile neighborhoods of the oncogenic epidermal growth factor receptor and orthogonally validated more than 20 neighbors using immunoassays and AlphaFold-Multimer prediction. We further profiled the protein neighborhoods of cell-cell synapses induced by bispecific T cell engagers and chimeric antigen receptor T cells. This integrated multiscale PLP platform maps local and distal protein networks on and between cell surfaces, which will aid in the systematic construction of the cell surface interactome, revealing horizontal signaling partners and reveal new immunotherapeutic opportunities.
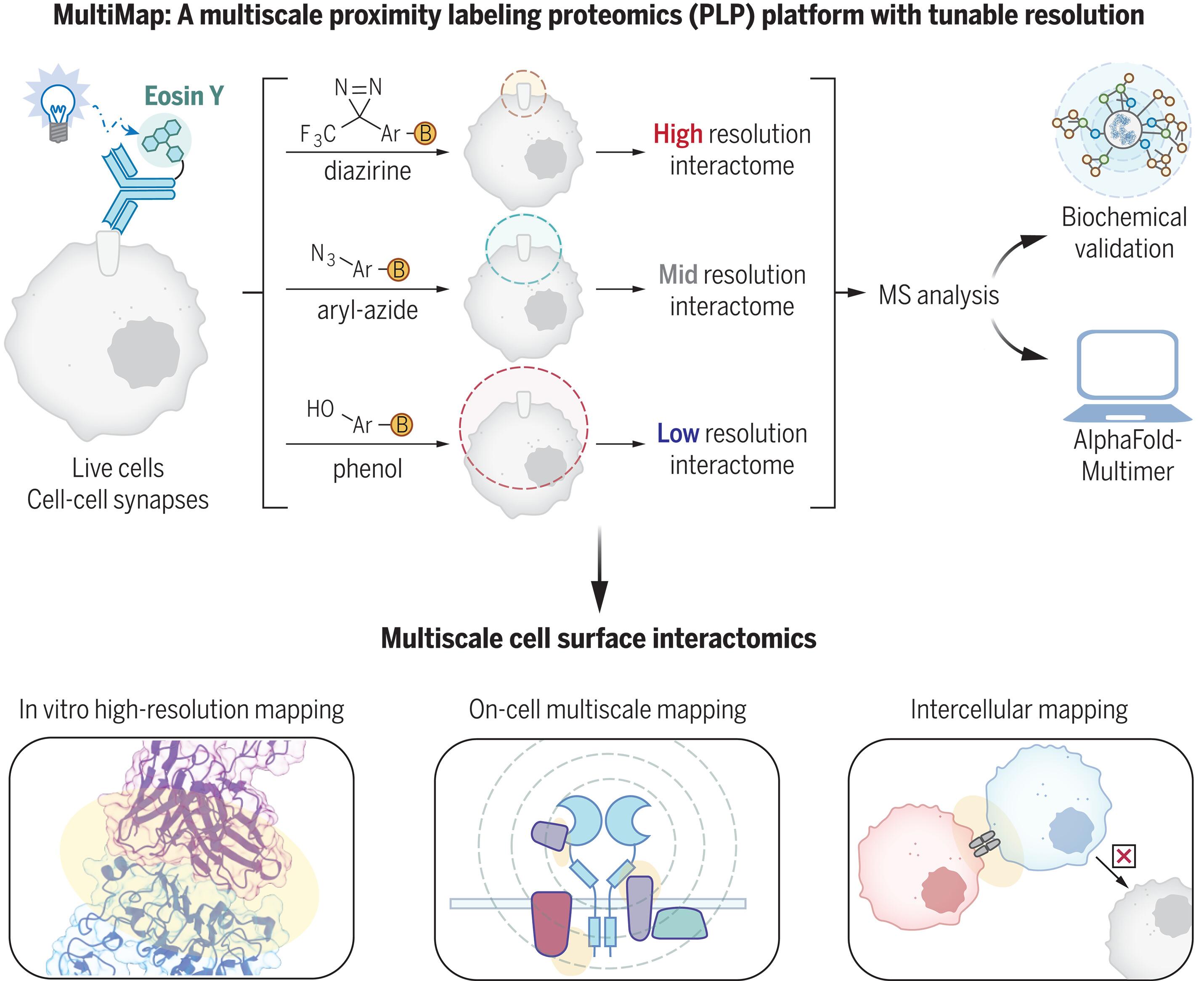
多尺度光催化接近标记揭示了细胞上和细胞间的细胞表面邻居。
近距离标记蛋白质组学(PLP)策略是获得蛋白质邻域快照的有力方法。在这里,我们介绍了一种分辨率可调的多尺度 PLP 方法,该方法使用了一种市售的光催化剂 Eosin Y,在可见光照射下可激活不同标记半径的光致发光物。我们将该平台用于分析致癌表皮生长因子受体的邻域,并利用免疫测定和 AlphaFold-Multimer 预测对 20 多个邻域进行了正交验证。我们进一步分析了由双特异性 T 细胞啮合因子和嵌合抗原受体 T 细胞诱导的细胞-细胞突触的蛋白质邻域。这个集成的多尺度 PLP 平台绘制了细胞表面和细胞表面之间的局部和远端蛋白质网络,这将有助于系统地构建细胞表面相互作用组,揭示水平信号传导伙伴,发现新的免疫治疗机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


