A metabolic atlas of blood cells in young and aged mice identifies uridine as a metabolite to rejuvenate aged hematopoietic stem cells
IF 17
Q1 CELL BIOLOGY
引用次数: 0
Abstract
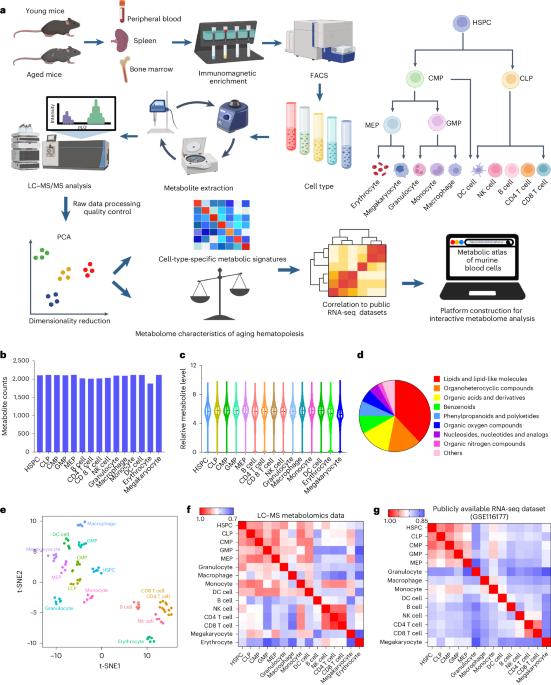
Aging of hematopoietic stem cells (HSCs) is accompanied by impaired self-renewal ability, myeloid skewing, immunodeficiencies and increased susceptibility to malignancies. Although previous studies highlighted the pivotal roles of individual metabolites in hematopoiesis, comprehensive and high-resolution metabolomic profiles of different hematopoietic cells across ages are still lacking. In this study, we created a metabolome atlas of different blood cells across ages in mice. We reveal here that purine, pyrimidine and retinol metabolism are enriched in young hematopoietic stem and progenitor cells (HSPCs), whereas glutamate and sphingolipid metabolism are concentrated in aged HSPCs. Through metabolic screening, we identified uridine as a potential regulator to rejuvenate aged HSPCs. Mechanistically, uridine treatment upregulates the FoxO signaling pathway and enhances self-renewal while suppressing inflammation in aged HSCs. Finally, we constructed an open-source platform for public easy access and metabolomic analysis in blood cells. Collectively, we provide a resource for metabolic studies in hematopoiesis that can contribute to future anti-aging metabolite screening. Zeng, Shi, Han, Hu, Li, Wei et al. present a metabolic atlas covering 15 hematopoietic cell types from young and aged mice. By screening metabolites that are depleted with age, they identify that uridine treatment can restore function in aged hematopoietic stem cells.
年轻和衰老小鼠血细胞代谢图谱发现尿苷是使衰老造血干细胞恢复活力的代谢物。
造血干细胞(HSCs)的衰老伴随着自我更新能力受损、髓系偏斜、免疫缺陷和对恶性肿瘤的易感性增加。尽管之前的研究强调了个别代谢物在造血过程中的关键作用,但目前仍缺乏不同年龄段造血细胞的全面、高分辨率代谢组学图谱。在这项研究中,我们绘制了小鼠各年龄段不同血细胞的代谢组图谱。我们在此发现,嘌呤、嘧啶和视黄醇代谢富集于年轻的造血干细胞和祖细胞(HSPCs),而谷氨酸和鞘脂代谢则集中于年老的HSPCs。通过代谢筛选,我们发现尿苷是一种潜在的调节剂,可使衰老的 HSPC 恢复活力。从机理上讲,尿苷处理可上调 FoxO 信号通路,在抑制炎症的同时增强衰老造血干细胞的自我更新能力。最后,我们构建了一个开源平台,方便公众访问并进行血细胞代谢组学分析。总之,我们为造血过程中的代谢研究提供了资源,有助于未来抗衰老代谢物的筛选。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


