The genetic architecture of biological age in nine human organ systems
IF 17
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Investigating the genetic underpinnings of human aging is essential for unraveling the etiology of and developing actionable therapies for chronic diseases. Here, we characterize the genetic architecture of the biological age gap (BAG; the difference between machine learning-predicted age and chronological age) across nine human organ systems in 377,028 participants of European ancestry from the UK Biobank. The BAGs were computed using cross-validated support vector machines, incorporating imaging, physical traits and physiological measures. We identify 393 genomic loci–BAG pairs (P < 5 × 10–8) linked to the brain, eye, cardiovascular, hepatic, immune, metabolic, musculoskeletal, pulmonary and renal systems. Genetic variants associated with the nine BAGs are predominantly specific to the respective organ system (organ specificity) while exerting pleiotropic links with other organ systems (interorgan cross-talk). We find that genetic correlation between the nine BAGs mirrors their phenotypic correlation. Further, a multiorgan causal network established from two-sample Mendelian randomization and latent causal variance models revealed potential causality between chronic diseases (for example, Alzheimer’s disease and diabetes), modifiable lifestyle factors (for example, sleep duration and body weight) and multiple BAGs. Our results illustrate the potential for improving human organ health via a multiorgan network, including lifestyle interventions and drug repurposing strategies. Using machine learning techniques applied to multimodal UK Biobank data, Wen et al. characterize the genetic basis of the biological age gaps of individual organs, uncovering interorgan cross-talk and links between chronic diseases, lifestyle factors and biological age gaps.
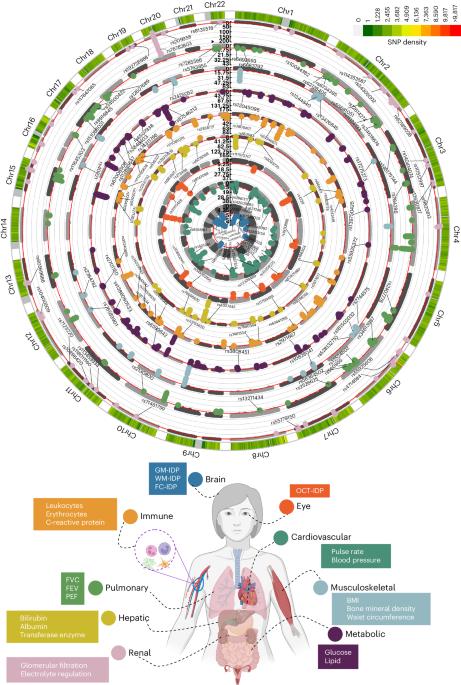
九个人体器官系统中生物年龄的基因结构。
研究人类衰老的遗传基础对于揭示慢性疾病的病因和开发可行的疗法至关重要。在这里,我们描述了英国生物库中 377,028 名具有欧洲血统的参与者的九个人体器官系统的生物年龄差距(BAG,即机器学习预测年龄与计时年龄之间的差异)的遗传结构。BAG 是通过交叉验证的支持向量机计算得出的,并结合了成像、身体特征和生理指标。我们确定了与大脑、眼睛、心血管、肝脏、免疫、新陈代谢、肌肉骨骼、肺部和肾脏系统相关的 393 个基因组位点-BAG 对(P -8)。与这九个 BAG 相关的基因变异主要针对各自的器官系统(器官特异性),同时与其他器官系统存在多效应联系(器官间交叉作用)。我们发现,九个 BAG 之间的遗传相关性反映了它们的表型相关性。此外,通过双样本孟德尔随机化和潜在因果方差模型建立的多器官因果网络揭示了慢性疾病(如阿尔茨海默病和糖尿病)、可改变的生活方式因素(如睡眠时间和体重)和多个 BAG 之间的潜在因果关系。我们的研究结果说明了通过多器官网络(包括生活方式干预和药物再利用策略)改善人体器官健康的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


