Metabolic inflexibility promotes mitochondrial health during liver regeneration
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Mitochondria are critical for proper organ function and mechanisms to promote mitochondrial health during regeneration would benefit tissue homeostasis. We report that during liver regeneration, proliferation is suppressed in electron transport chain (ETC)–dysfunctional hepatocytes due to an inability to generate acetyl-CoA from peripheral fatty acids through mitochondrial β-oxidation. Alternative modes for acetyl-CoA production from pyruvate or acetate are suppressed in the setting of ETC dysfunction. This metabolic inflexibility forces a dependence on ETC-functional mitochondria and restoring acetyl-CoA production from pyruvate is sufficient to allow ETC-dysfunctional hepatocytes to proliferate. We propose that metabolic inflexibility within hepatocytes can be advantageous by limiting the expansion of ETC-dysfunctional cells.
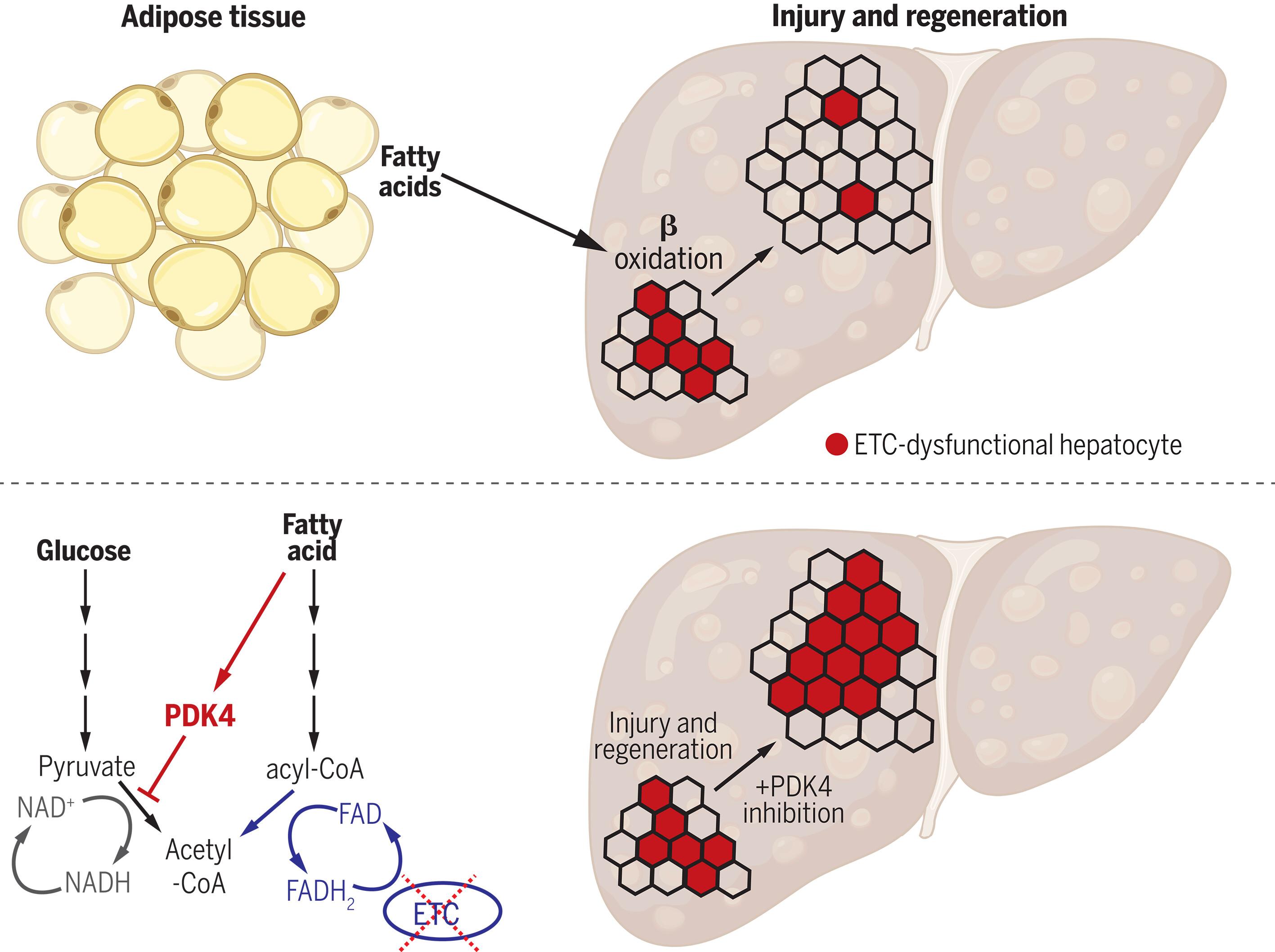
新陈代谢不灵活促进肝脏再生过程中的线粒体健康
线粒体对器官的正常功能至关重要,在再生过程中促进线粒体健康的机制将有利于组织的稳态。我们报告说,在肝脏再生过程中,电子传递链(ETC)功能障碍的肝细胞由于无法通过线粒体β-氧化从外周脂肪酸生成乙酰-CoA,增殖受到抑制。在 ETC 功能障碍的情况下,从丙酮酸或乙酸产生乙酰-CoA 的替代模式受到抑制。这种新陈代谢的不灵活性迫使人们对 ETC 功能线粒体产生依赖,而恢复丙酮酸产生乙酰-CoA 就足以让 ETC 功能障碍的肝细胞增殖。我们认为,肝细胞内的新陈代谢不灵活可以限制 ETC 功能失调细胞的增殖,因而是有利的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


