The neuropathobiology of multiple sclerosis
IF 28.7
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
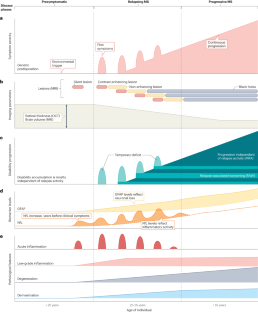
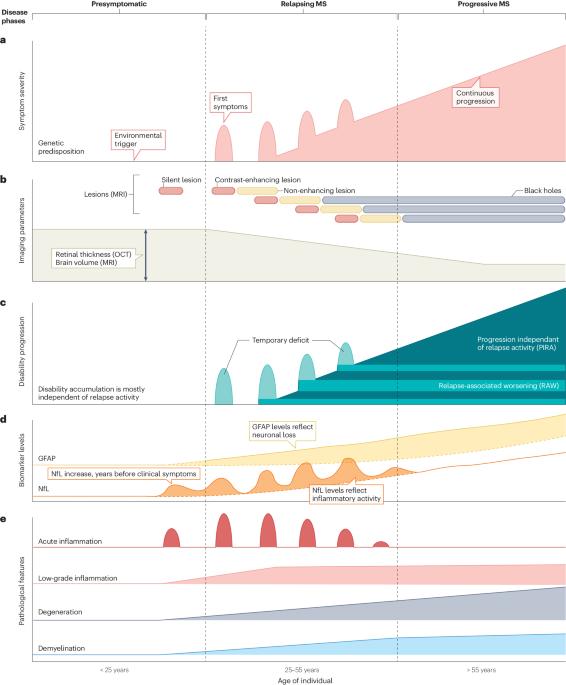
Chronic low-grade inflammation and neuronal deregulation are two components of a smoldering disease activity that drives the progression of disability in people with multiple sclerosis (MS). Although several therapies exist to dampen the acute inflammation that drives MS relapses, therapeutic options to halt chronic disability progression are a major unmet clinical need. The development of such therapies is hindered by our limited understanding of the neuron-intrinsic determinants of resilience or vulnerability to inflammation. In this Review, we provide a neuron-centric overview of recent advances in deciphering neuronal response patterns that drive the pathology of MS. We describe the inflammatory CNS environment that initiates neurotoxicity by imposing ion imbalance, excitotoxicity and oxidative stress, and by direct neuro-immune interactions, which collectively lead to mitochondrial dysfunction and epigenetic dysregulation. The neuronal demise is further amplified by breakdown of neuronal transport, accumulation of cytosolic proteins and activation of cell death pathways. Continuous neuronal damage perpetuates CNS inflammation by activating surrounding glia cells and by directly exerting toxicity on neighbouring neurons. Further, we explore strategies to overcome neuronal deregulation in MS and compile a selection of neuronal actuators shown to impact neurodegeneration in preclinical studies. We conclude by discussing the therapeutic potential of targeting such neuronal actuators in MS, including some that have already been tested in interventional clinical trials. Slowing neurodegeneration is the most pressing clinical need for multiple sclerosis (MS). In this Review, Woo, Engler and Friese provide a neuron-centric view on inflammation-induced neurodegeneration in MS and discuss key pathways and molecules that can be therapeutically targeted.
多发性硬化症的神经生物学
慢性低度炎症和神经元失调是导致多发性硬化症(MS)患者残疾进展的烟雾性疾病活动的两个组成部分。虽然已有多种疗法可以抑制导致多发性硬化复发的急性炎症,但阻止慢性残疾进展的治疗方案仍是一项尚未满足的重大临床需求。我们对神经元内在决定炎症恢复力或脆弱性的因素了解有限,这阻碍了此类疗法的开发。在这篇综述中,我们以神经元为中心概述了在破译驱动多发性硬化症病理的神经元反应模式方面的最新进展。我们描述了中枢神经系统的炎症环境,这种环境通过强加离子失衡、兴奋毒性和氧化应激以及直接的神经-免疫相互作用引发神经毒性,共同导致线粒体功能障碍和表观遗传失调。神经元转运功能的破坏、细胞膜蛋白的积累和细胞死亡途径的激活进一步加剧了神经元的衰亡。神经元的持续损伤会激活周围的神经胶质细胞,并直接对邻近的神经元产生毒性,从而使中枢神经系统炎症长期存在。此外,我们还探讨了克服多发性硬化症神经元失调的策略,并汇编了在临床前研究中被证明可影响神经退行性变的神经元致动器。最后,我们讨论了针对多发性硬化症神经元致动器的治疗潜力,包括一些已经在干预性临床试验中进行测试的神经元致动器。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Neuroscience
NEUROSCIENCES-
自引率
0.60%
发文量
104
期刊介绍:
Nature Reviews Neuroscience is a multidisciplinary journal that covers various fields within neuroscience, aiming to offer a comprehensive understanding of the structure and function of the central nervous system. Advances in molecular, developmental, and cognitive neuroscience, facilitated by powerful experimental techniques and theoretical approaches, have made enduring neurobiological questions more accessible. Nature Reviews Neuroscience serves as a reliable and accessible resource, addressing the breadth and depth of modern neuroscience. It acts as an authoritative and engaging reference for scientists interested in all aspects of neuroscience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


