Total Synthesis and Antibacterial Activity of Marine Tris-indole Alkaloid Tulongicin A
IF 3.3
2区 生物学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
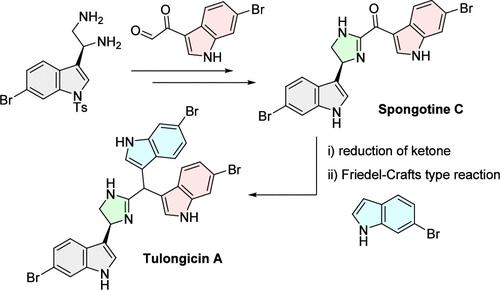
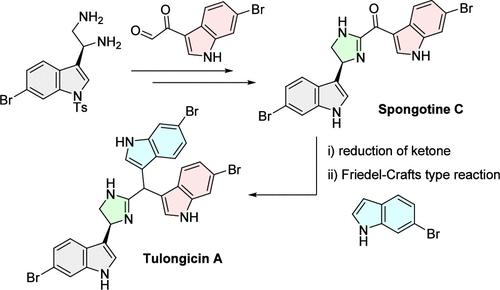
Bis-indole alkaloids from marine sponges are an intriguing class of natural products with a variety of activities. However, only a preliminary biological study of tulongicin A (5), a related previously isolated marine tris-indole alkaloid, has been conducted. In this study, we accomplished the first asymmetric total synthesis of 5 via the construction of an imidazoline-linked bis-indolylmethane skeleton using a Friedel–Crafts-type reaction. Our synthesis enabled a detailed study of the antibacterial profile of 5. Compound 5 displayed bactericidal activity against Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA) strains.
海洋三吲哚类生物碱 Tulongicin A 的全合成及其抗菌活性
海洋海绵中的双吲哚生物碱是一类具有多种活性的令人感兴趣的天然产物。然而,对于之前分离出的一种相关的海洋三吲哚生物碱--土龙毒素 A(5),目前只进行了初步的生物学研究。在本研究中,我们利用弗里德尔-卡夫斯(Friedel-Crafts)型反应,通过构建咪唑啉连接的双吲哚甲烷骨架,首次完成了 5 的不对称全合成。通过我们的合成,对 5 的抗菌特性进行了详细研究。化合物 5 对金黄色葡萄球菌(包括耐甲氧西林金黄色葡萄球菌(MRSA)菌株)具有杀菌活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


