Total Syntheses of the Structures Assigned to Denigrins A, B, C, F, and G, 3,4-Diaryl-Pyrrole and -Pyrrolidinone Alkaloids, and the Conversion of Congener B into the Co-metabolite Spirodactylone
IF 3.3
2区 生物学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The title marine natural products have been prepared by total synthesis and in the case of congeners 3, 6, and 7 for the first time. Each of these was obtained by manipulation of readily prepared denigrin B (2). The structure, 3, assigned to denigrin C is shown to be incorrect. Reaction of compound 2 with DDQ has led, in high yield, to the related natural product spirodactylone (16), while treating the corresponding permethyl ether 15 with PIFA/BF3·Et2O provides compound 20, embodying an isomeric framework.
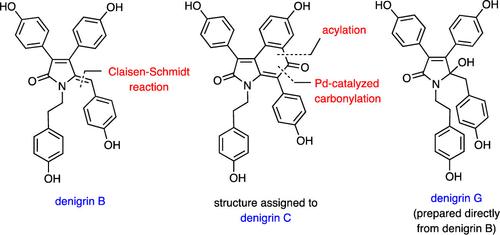
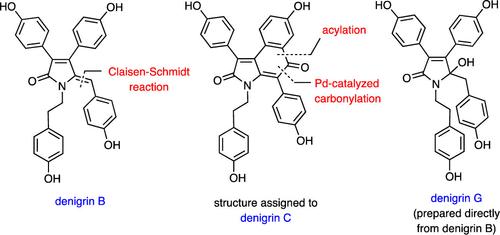
Denigrins A、B、C、F 和 G、3,4-二芳基吡咯和吡咯烷酮生物碱结构的全合成,以及同系物 B 转化为共代谢物螺内酯。
上述海洋天然产物是通过全合成方法制备的,其中同系物 3、6 和 7 是首次制备。每种同系物都是通过操作容易制备的变性变色菊酯 B (2) 而获得的。3 被认为是变性木酚 C 的结构被证明是不正确的。将化合物 2 与 DDQ 反应,可以高产率地得到相关的天然产物螺内酯(16),而将相应的高甲基醚 15 与 PIFA/BF3-Et2O 处理,可以得到化合物 20,其中包含一个异构体框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


