Reactivities of acrylamide warheads toward cysteine targets: a QM/ML approach to covalent inhibitor design
Abstract
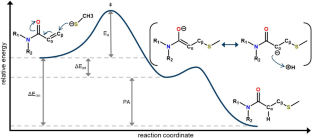
Covalent inhibition offers many advantages over non-covalent inhibition, but covalent warhead reactivity must be carefully balanced to maintain potency while avoiding unwanted side effects. While warhead reactivities are commonly measured with assays, a computational model to predict warhead reactivities could be useful for several aspects of the covalent inhibitor design process. Studies have shown correlations between covalent warhead reactivities and quantum mechanic (QM) properties that describe important aspects of the covalent reaction mechanism. However, the models from these studies are often linear regression equations and can have limitations associated with their usage. Applications of machine learning (ML) models to predict covalent warhead reactivities with QM descriptors are not extensively seen in the literature. This study uses QM descriptors, calculated at different levels of theory, to train ML models to predict reactivities of covalent acrylamide warheads. The QM/ML models are compared with linear regression models built upon the same QM descriptors and with ML models trained on structure-based features like Morgan fingerprints and RDKit descriptors. Experiments show that the QM/ML models outperform the linear regression models and the structure-based ML models, and literature test sets demonstrate the power of the QM/ML models to predict reactivities of unseen acrylamide warhead scaffolds. Ultimately, these QM/ML models are effective, computationally feasible tools that can expedite the design of new covalent inhibitors.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


