A blueprint for tumor-infiltrating B cells across human cancers
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
B lymphocytes are essential mediators of humoral immunity and play multiple roles in human cancer. To decode the functions of tumor-infiltrating B cells, we generated a B cell blueprint encompassing single-cell transcriptome, B cell–receptor repertoire, and chromatin accessibility data across 20 different cancer types (477 samples, 269 patients). B cells harbored extraordinary heterogeneity and comprised 15 subsets, which could be grouped into two independent developmental paths (extrafollicular versus germinal center). Tumor types grouped into the extrafollicular pathway were linked with worse clinical outcomes and resistance to immunotherapy. The dysfunctional extrafollicular program was associated with glutamine-derived metabolites through epigenetic-metabolic cross-talk, which promoted a T cell–driven immunosuppressive program. These data suggest an intratumor B cell balance between extrafollicular and germinal-center responses and suggest that humoral immunity could possibly be harnessed for B cell–targeting immunotherapy.
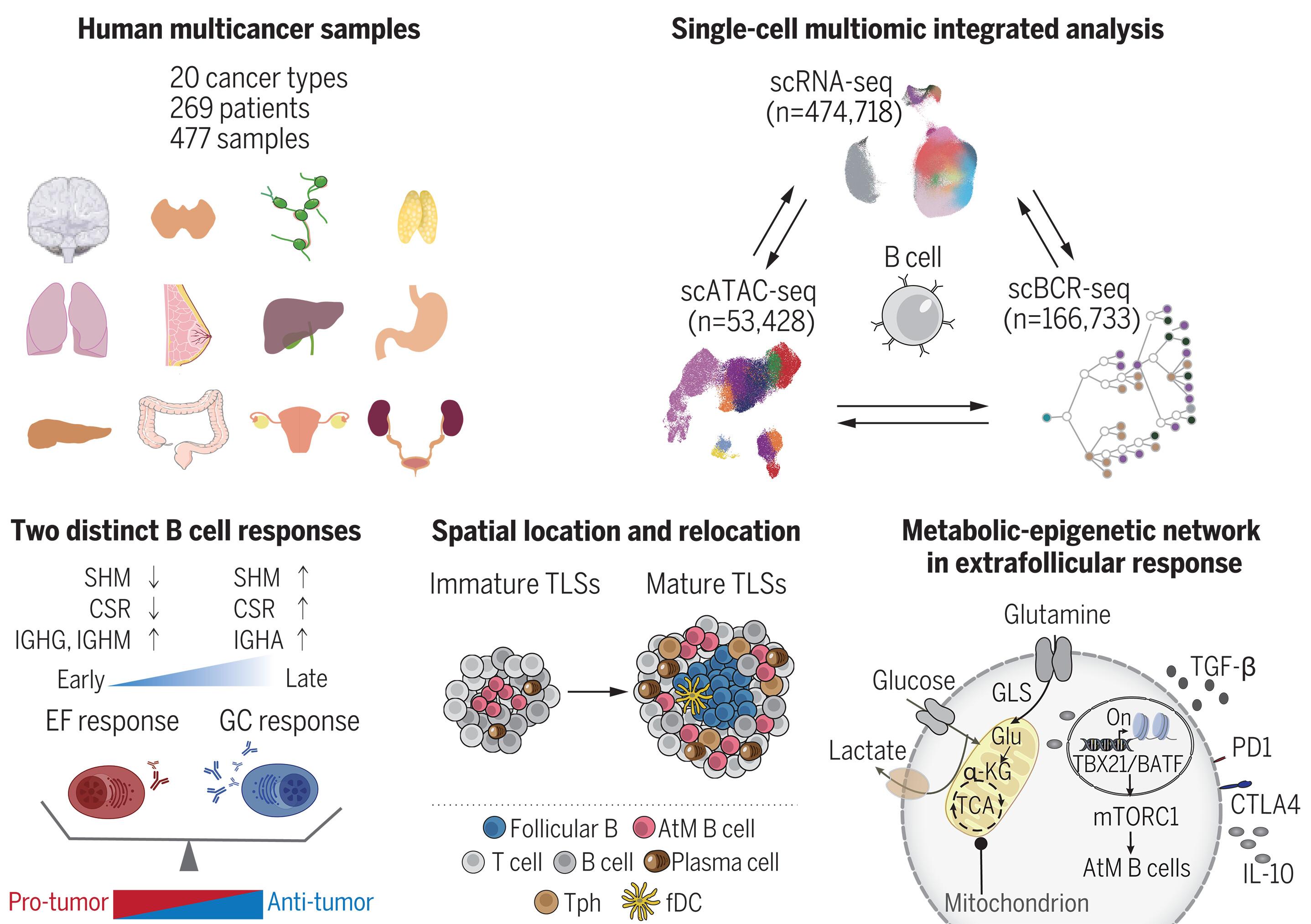
人类癌症中肿瘤浸润 B 细胞的蓝图
B 淋巴细胞是体液免疫的重要介质,在人类癌症中发挥着多种作用。为了解读肿瘤浸润 B 细胞的功能,我们生成了一个 B 细胞蓝图,其中包括 20 种不同癌症类型(477 个样本,269 名患者)的单细胞转录组、B 细胞受体谱系和染色质可及性数据。B细胞具有非同寻常的异质性,由15个亚群组成,可分为两种独立的发育途径(滤泡外与生殖中心)。被归入卵泡外途径的肿瘤类型与较差的临床疗效和对免疫疗法的抗药性有关。功能失调的小叶外程序通过表观遗传-代谢交叉对话与谷氨酰胺衍生代谢物有关,这促进了T细胞驱动的免疫抑制程序。这些数据表明了肿瘤内B细胞在卵泡外和生殖中心反应之间的平衡,并表明体液免疫有可能被用于B细胞靶向免疫疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


