Total Synthesis of a TNBC-Selective Cytotoxic Bromo Nor-eremophilane, PC-A, and Its Preliminary Structure–Activity Relationships
IF 3.3
2区 生物学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
PC-A (1), a bromo nor-eremophilane, showed selective antiproliferative activity against a triple-negative breast cancer (TNBC) cell line. This unique activity prompted us to establish a total synthesis to facilitate a structure–activity relationship (SAR) study and selectivity optimization. An enantioselective first total synthesis of 1 was achieved starting from (R)-carvone through a side chain extension with a Mukaiyama aldol reaction and decalin construction. The synthesized decalin derivatives and debromo PC-A (2) were evaluated for antiproliferative activity against five human tumor cell lines, including TNBC, to assess preliminary SAR correlations.
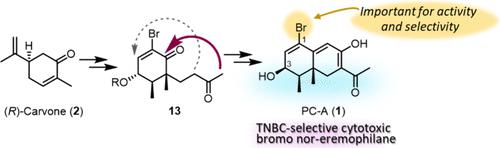
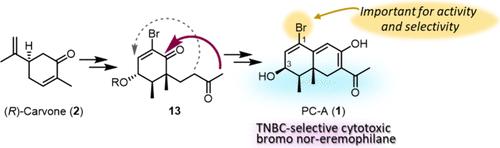
TNBC 选择性细胞毒性溴代异壬烷 PC-A 的全合成及其初步结构-活性关系。
PC-A (1)是一种溴代nor-eremophilane,对三阴性乳腺癌(TNBC)细胞系具有选择性抗增殖活性。这种独特的活性促使我们建立一种全合成方法,以促进结构-活性关系(SAR)研究和选择性优化。我们以 (R)- 香芹酮为起点,通过 Mukaiyama 醛醇反应和蜕皮激素构建侧链延伸,首次实现了 1 的对映选择性全合成。研究人员评估了合成的癸醛衍生物和脱溴 PC-A (2) 对包括 TNBC 在内的五种人类肿瘤细胞系的抗增殖活性,以评估初步的 SAR 关联性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


