Cell type-specific roles of APOE4 in Alzheimer disease
IF 28.7
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
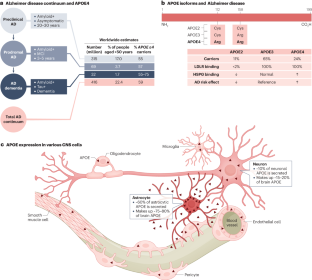
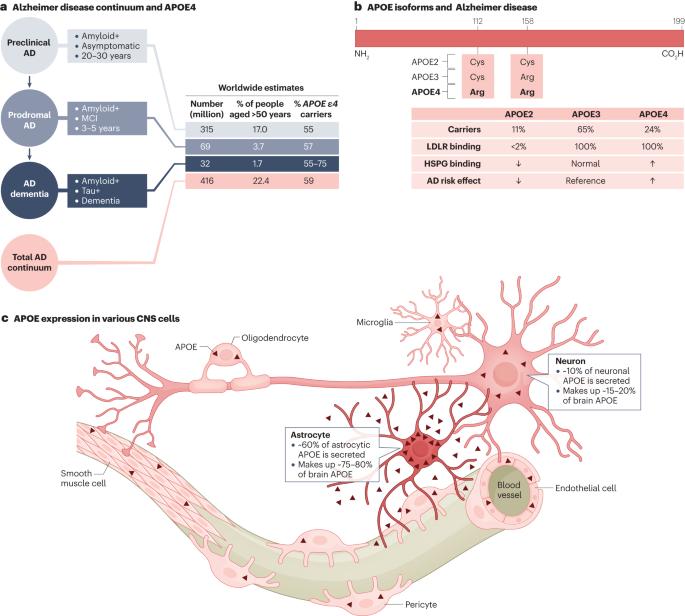
The ɛ4 allele of the apolipoprotein E gene (APOE), which translates to the APOE4 isoform, is the strongest genetic risk factor for late-onset Alzheimer disease (AD). Within the CNS, APOE is produced by a variety of cell types under different conditions, posing a challenge for studying its roles in AD pathogenesis. However, through powerful advances in research tools and the use of novel cell culture and animal models, researchers have recently begun to study the roles of APOE4 in AD in a cell type-specific manner and at a deeper and more mechanistic level than ever before. In particular, cutting-edge omics studies have enabled APOE4 to be studied at the single-cell level and have allowed the identification of critical APOE4 effects in AD-vulnerable cellular subtypes. Through these studies, it has become evident that APOE4 produced in various types of CNS cell — including astrocytes, neurons, microglia, oligodendrocytes and vascular cells — has diverse roles in AD pathogenesis. Here, we review these scientific advances and propose a cell type-specific APOE4 cascade model of AD. In this model, neuronal APOE4 emerges as a crucial pathological initiator and driver of AD pathogenesis, instigating glial responses and, ultimately, neurodegeneration. In addition, we provide perspectives on future directions for APOE4 research and related therapeutic developments in the context of AD. Within the CNS, APOE4 — a risk factor for late-onset Alzheimer disease — is produced by a variety of cell types. Blumenfeld, Yip, Kim and Huang discuss recent scientific advances that have begun to unravel the cell type-specific roles of APOE4 and outline a corresponding cell type-specific APOE4 cascade model of Alzheimer disease.
APOE4 在阿尔茨海默病中的细胞特异性作用
载脂蛋白 E 基因(APOE)的ɛ4 等位基因转化为 APOE4 同工型,是晚发性阿尔茨海默病(AD)的最强遗传风险因素。在中枢神经系统内,APOE 在不同条件下由多种细胞类型产生,这给研究其在阿尔茨海默病发病机制中的作用带来了挑战。然而,通过研究工具的强大进步以及新型细胞培养和动物模型的使用,研究人员最近开始以细胞类型特异性的方式,在比以往更深入、更机制化的水平上研究 APOE4 在 AD 中的作用。特别是,最前沿的全息研究使 APOE4 得以在单细胞水平上进行研究,并确定了 APOE4 在 AD 易感细胞亚型中的关键作用。通过这些研究,我们发现在中枢神经系统各类细胞(包括星形胶质细胞、神经元、小胶质细胞、少突胶质细胞和血管细胞)中产生的APOE4在AD发病机制中发挥着不同的作用。在此,我们回顾了这些科学进展,并提出了一种细胞类型特异性 APOE4 级联的 AD 模型。在这一模型中,神经元 APOE4 成为 AD 发病机制的关键病理启动因子和驱动因子,引发神经胶质细胞反应,最终导致神经退行性变。此外,我们还对APOE4研究的未来方向和AD相关疗法的发展进行了展望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Neuroscience
NEUROSCIENCES-
自引率
0.60%
发文量
104
期刊介绍:
Nature Reviews Neuroscience is a multidisciplinary journal that covers various fields within neuroscience, aiming to offer a comprehensive understanding of the structure and function of the central nervous system. Advances in molecular, developmental, and cognitive neuroscience, facilitated by powerful experimental techniques and theoretical approaches, have made enduring neurobiological questions more accessible. Nature Reviews Neuroscience serves as a reliable and accessible resource, addressing the breadth and depth of modern neuroscience. It acts as an authoritative and engaging reference for scientists interested in all aspects of neuroscience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


