Dietary polyphenols reduced the allergenicity of β-lactoglobulin via non-covalent interactions: a study on the structure-allergenicity relationship
IF 7.4
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Studies showed that complexation of polyphenols with milk allergens reduced their immunogenic potential. However, the relationship between structures of polyphenols and their hypoallergenic effects on milk allergens in association with physiological and conformational changes of the complexes remain unclear. In this study, polyphenols from eight botanical sources were extracted to prepare non-covalent complexes with β-lactoglobulin (β-LG), a major allergen in milk. The dominant phenolic compounds bound to β-LG with a diminished allergenicity were identified to investigate their respective role on the structural and allergenic properties of β-LG. Extracts from Vaccinium fruits and black soybeans were found to have great inhibitory effects on the IgE- and IgG-binding abilities of β-LG. Among the fourteen structure-related phenolic compounds, flavonoids and tannins with larger MWs and multi-hydroxyl substituents, notably rutin, EGCG, and ellagitannins were more potent to elicit changes on the conformational structures of β-LG to decrease the allergenicity of complexed β-LG. Correlation analysis further demonstrated that a destabilized secondary structure and protein depolymerization caused by polyphenol-binding were closely related to the allergenicity property of formed complexes. This study provides insights into the understanding of structure-allergenicity relationship of β-LG-polyphenol interactions and would benefit the development of polyphenol-fortified matrices with hypoallergenic potential.
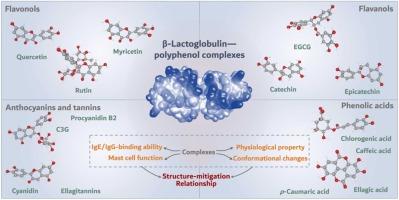
膳食多酚通过非共价相互作用降低了β-乳球蛋白的致敏性:关于结构-致敏性关系的研究
研究表明,多酚与牛奶过敏原的复合物可降低其免疫原性。然而,多酚的结构及其对牛奶过敏原的低过敏效应与复合物的生理和构象变化之间的关系仍不清楚。本研究从八种植物中提取多酚,制备与牛奶中主要过敏原 β-乳球蛋白(β-LG)的非共价复合物。研究人员确定了与β-LG结合后过敏性降低的主要酚类化合物,以研究它们各自对β-LG的结构和过敏特性所起的作用。研究发现,从越橘果实和黑大豆中提取的提取物对 β-LG 的 IgE 和 IgG 结合能力有很大的抑制作用。在 14 种结构相关的酚类化合物中,具有较大分子量和多羟基取代基的黄酮类和单宁类化合物,尤其是芦丁、EGCG 和鞣花丹宁类化合物,能更有效地引起 β-LG 构象结构的变化,从而降低与 β-LG 复合物的致敏性。相关分析进一步表明,多酚结合导致的二级结构不稳定和蛋白质解聚与形成的复合物的致敏性密切相关。这项研究为了解β-LG-多酚相互作用的结构-致敏性关系提供了深入的见解,有利于开发具有低致敏潜力的多酚强化基质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Science and Human Wellness
Agricultural and Biological Sciences-Food Science
CiteScore
8.30
自引率
5.70%
发文量
80
审稿时长
28 days
期刊介绍:
Food Science and Human Wellness is an international peer-reviewed journal that provides a forum for the dissemination of the latest scientific results in food science, nutriology, immunology and cross-field research. Articles must present information that is novel, has high impact and interest, and is of high scientific quality. By their effort, it has been developed to promote the public awareness on diet, advocate healthy diet, reduce the harm caused by unreasonable dietary habit, and directs healthy food development for food industrial producers.
文献相关原料
公司名称
产品信息
上海源叶
Procyanidin B2 (PB2)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


