适用于信息丰富、密集数据的最佳样本选择。
IF 2.2
4区 医学
Q3 PHARMACOLOGY & PHARMACY
Journal of Pharmacokinetics and Pharmacodynamics
Pub Date : 2024-02-01
Epub Date: 2023-08-10
DOI:10.1007/s10928-023-09883-7
引用次数: 0
摘要
密集数据可分为信息贫乏的超密集数据(第 1 类密集数据)和信息丰富的密集数据(第 2 类密集数据)。可对第 1 类密集数据进行任意、随机或优化稀疏处理,以尽量减少计算负担和统计问题(如自相关性)。相比之下,前瞻性或回顾性优化设计可应用于第二类密集数据,以便利用有限的资源(资金和/或时间)获得最大的信息收益。在此,我们介绍一种回顾性优化选择策略,用于从一组已测定总药物浓度的离散血浆样本中量化非结合药物浓度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
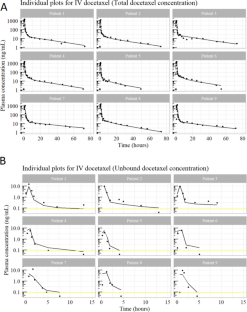
Optimal sample selection applied to information rich, dense data.
Dense data can be classified into superdense information-poor data (type 1 dense data) and dense information-rich data (type 2 dense data). Arbitrary, random, or optimal thinning may be applied to type 1 dense data to minimise computational burden and statistical issues (such as autocorrelation). In contrast, a prospective or retrospective optimal design can be applied to type 2 dense data to maximise information gain from limited resources (capital and/or time). Here we describe a retrospective optimal selection strategy for quantification of unbound drug concentration from a discrete set of plasma samples where the total drug concentration has been measured.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.90
自引率
4.00%
发文量
39
审稿时长
6-12 weeks
期刊介绍:
Broadly speaking, the Journal of Pharmacokinetics and Pharmacodynamics covers the area of pharmacometrics. The journal is devoted to illustrating the importance of pharmacokinetics, pharmacodynamics, and pharmacometrics in drug development, clinical care, and the understanding of drug action. The journal publishes on a variety of topics related to pharmacometrics, including, but not limited to, clinical, experimental, and theoretical papers examining the kinetics of drug disposition and effects of drug action in humans, animals, in vitro, or in silico; modeling and simulation methodology, including optimal design; precision medicine; systems pharmacology; and mathematical pharmacology (including computational biology, bioengineering, and biophysics related to pharmacology, pharmacokinetics, orpharmacodynamics). Clinical papers that include population pharmacokinetic-pharmacodynamic relationships are welcome. The journal actively invites and promotes up-and-coming areas of pharmacometric research, such as real-world evidence, quality of life analyses, and artificial intelligence. The Journal of Pharmacokinetics and Pharmacodynamics is an official journal of the International Society of Pharmacometrics.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


