Vanessa Dubois, Silvia Ciancia, Stefanie Doms, Sarah El Kharraz, Vera Sommers, Na Ri Kim, Karel David, Jolien Van Dijck, Roger Valle-Tenney, Christa Maes, Leen Antonio, Brigitte Decallonne, Geert Carmeliet, Frank Claessens, Martine Cools, Dirk Vanderschueren
求助PDF
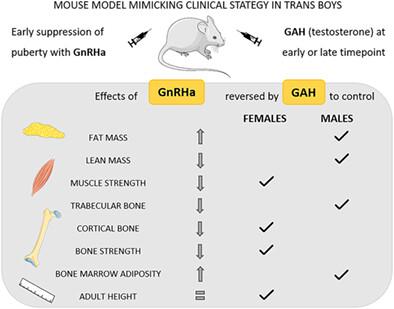
{"title":"睾酮在模仿跨性别男孩临床策略的小鼠模型中恢复青春期早期抑制后的身体成分、骨量和骨强度","authors":"Vanessa Dubois, Silvia Ciancia, Stefanie Doms, Sarah El Kharraz, Vera Sommers, Na Ri Kim, Karel David, Jolien Van Dijck, Roger Valle-Tenney, Christa Maes, Leen Antonio, Brigitte Decallonne, Geert Carmeliet, Frank Claessens, Martine Cools, Dirk Vanderschueren","doi":"10.1002/jbmr.4832","DOIUrl":null,"url":null,"abstract":"<div>\n \n <p>Transgender youth increasingly present at pediatric gender services. Some of them receive long-term puberty suppression with gonadotropin-releasing hormone analogues (GnRHa) before starting gender-affirming hormones (GAH). The impact of GnRHa use started in early puberty on bone composition and bone mass accrual is unexplored. It is furthermore unclear whether subsequent GAH fully restore GnRHa effects and whether the timing of GAH introduction matters. To answer these questions, we developed a mouse model mimicking the clinical strategy applied in trans boys. Prepubertal 4-week-old female mice were treated with GnRHa alone or with GnRHa supplemented with testosterone (T) from 6 weeks (early puberty) or 8 weeks (late puberty) onward. Outcomes were analyzed at 16 weeks and compared with untreated mice of both sexes. GnRHa markedly increased total body fat mass, decreased lean body mass, and had a modest negative impact on grip strength. Both early and late T administration shaped body composition to adult male levels, whereas grip strength was restored to female values. GnRHa-treated animals showed lower trabecular bone volume and reduced cortical bone mass and strength. These changes were reversed by T to female levels (cortical bone mass and strength) irrespective of the time of administration or even fully up to adult male control values (trabecular parameters) in case of earlier T start. The lower bone mass in GnRHa-treated mice was associated with increased bone marrow adiposity, also reversed by T. In conclusion, prolonged GnRHa use started in prepubertal female mice modifies body composition toward more fat and less lean mass and impairs bone mass acquisition and strength. Subsequent T administration counteracts GnRHa impact on these parameters, shaping body composition and trabecular parameters to male values while restoring cortical bone architecture and strength up to female but not male control levels. These findings could help guide clinical strategies in transgender care. © 2023 American Society for Bone and Mineral Research (ASBMR).</p>\n </div>","PeriodicalId":185,"journal":{"name":"Journal of Bone and Mineral Research","volume":"38 10","pages":"1497-1508"},"PeriodicalIF":5.1000,"publicationDate":"2023-05-24","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"3","resultStr":"{\"title\":\"Testosterone Restores Body Composition, Bone Mass, and Bone Strength Following Early Puberty Suppression in a Mouse Model Mimicking the Clinical Strategy in Trans Boys\",\"authors\":\"Vanessa Dubois, Silvia Ciancia, Stefanie Doms, Sarah El Kharraz, Vera Sommers, Na Ri Kim, Karel David, Jolien Van Dijck, Roger Valle-Tenney, Christa Maes, Leen Antonio, Brigitte Decallonne, Geert Carmeliet, Frank Claessens, Martine Cools, Dirk Vanderschueren\",\"doi\":\"10.1002/jbmr.4832\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<div>\\n \\n <p>Transgender youth increasingly present at pediatric gender services. Some of them receive long-term puberty suppression with gonadotropin-releasing hormone analogues (GnRHa) before starting gender-affirming hormones (GAH). The impact of GnRHa use started in early puberty on bone composition and bone mass accrual is unexplored. It is furthermore unclear whether subsequent GAH fully restore GnRHa effects and whether the timing of GAH introduction matters. To answer these questions, we developed a mouse model mimicking the clinical strategy applied in trans boys. Prepubertal 4-week-old female mice were treated with GnRHa alone or with GnRHa supplemented with testosterone (T) from 6 weeks (early puberty) or 8 weeks (late puberty) onward. Outcomes were analyzed at 16 weeks and compared with untreated mice of both sexes. GnRHa markedly increased total body fat mass, decreased lean body mass, and had a modest negative impact on grip strength. Both early and late T administration shaped body composition to adult male levels, whereas grip strength was restored to female values. GnRHa-treated animals showed lower trabecular bone volume and reduced cortical bone mass and strength. These changes were reversed by T to female levels (cortical bone mass and strength) irrespective of the time of administration or even fully up to adult male control values (trabecular parameters) in case of earlier T start. The lower bone mass in GnRHa-treated mice was associated with increased bone marrow adiposity, also reversed by T. In conclusion, prolonged GnRHa use started in prepubertal female mice modifies body composition toward more fat and less lean mass and impairs bone mass acquisition and strength. Subsequent T administration counteracts GnRHa impact on these parameters, shaping body composition and trabecular parameters to male values while restoring cortical bone architecture and strength up to female but not male control levels. These findings could help guide clinical strategies in transgender care. © 2023 American Society for Bone and Mineral Research (ASBMR).</p>\\n </div>\",\"PeriodicalId\":185,\"journal\":{\"name\":\"Journal of Bone and Mineral Research\",\"volume\":\"38 10\",\"pages\":\"1497-1508\"},\"PeriodicalIF\":5.1000,\"publicationDate\":\"2023-05-24\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"\",\"citationCount\":\"3\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Journal of Bone and Mineral Research\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/jbmr.4832\",\"RegionNum\":1,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ENDOCRINOLOGY & METABOLISM\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Journal of Bone and Mineral Research","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbmr.4832","RegionNum":1,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 3
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


