用于早期诊断癌症和异体移植排斥反应的肾脏可清除聚荧光团纳米传感器
IF 37.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 32
摘要
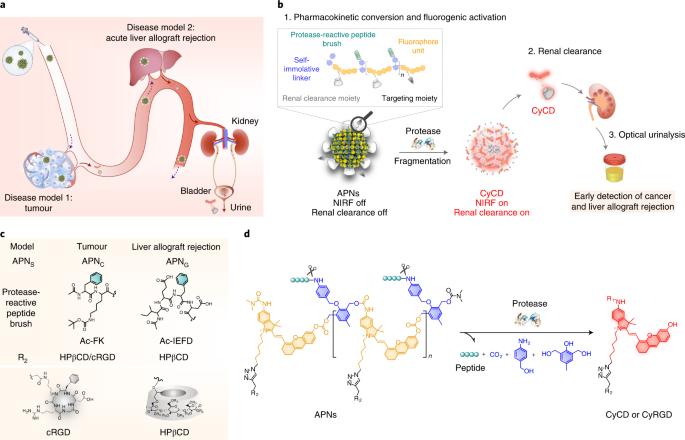
光学纳米粒子是一种前景广阔的诊断工具;然而,由于其光学成像深度较浅,从体内清除的速度较慢,阻碍了其在体内疾病检测中的应用。为了解决这些局限性,我们开发了可激活的聚荧光团纳米传感器,该传感器具有生物标记物触发的纳米粒子到分子的药代动力学转换和近红外荧光导通响应。可激活的聚荧光团纳米传感器可在疾病部位积聚,并与疾病相关的蛋白酶发生反应,在酶催化下发生原位解聚。这种疾病特异性相互作用可从可激活的聚荧光团纳米传感器中释放出肾脏可清除的荧光片段,用于非侵入性纵向尿液分析,其灵敏度和特异性可与侵入性活检和流式细胞术分析相媲美,优于黄金标准的血液和尿液分析。在啮齿动物模型中,可激活的聚荧光团纳米传感器能够超灵敏地检测肿瘤(直径 1.6 毫米)和早期诊断急性肝脏异体移植排斥反应。我们预计,我们的模块化纳米传感器平台可以通过简单的尿检对一系列疾病进行早期诊断。早期癌症检测通常需要进行侵入性活检。在这里,作者设计的纳米传感器可在体内被疾病相关酶解聚,产生荧光尿液信号,用于非侵入性早期诊断。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Renal clearable polyfluorophore nanosensors for early diagnosis of cancer and allograft rejection
Optical nanoparticles are promising diagnostic tools; however, their shallow optical imaging depth and slow clearance from the body have impeded their use for in vivo disease detection. To address these limitations, we develop activatable polyfluorophore nanosensors with biomarker-triggered nanoparticle-to-molecule pharmacokinetic conversion and near-infrared fluorogenic turn-on response. Activatable polyfluorophore nanosensors can accumulate at the disease site and react with disease-associated proteases to undergo in situ enzyme-catalysed depolymerization. This disease-specific interaction liberates renal-clearable fluorogenic fragments from activatable polyfluorophore nanosensors for non-invasive longitudinal urinalysis and outperforms the gold standard blood and urine assays, providing a level of sensitivity and specificity comparable to those of invasive biopsy and flow cytometry analysis. In rodent models, activatable polyfluorophore nanosensors enable ultrasensitive detection of tumours (1.6 mm diameter) and early diagnosis of acute liver allograft rejection. We anticipate that our modular nanosensor platform may be applied for early diagnosis of a range of diseases via a simple urine test. Early cancer detection typically involves invasive biopsies. Here the authors designed nanosensors that are depolymerized by disease-associated enzymes in vivo to produce fluorescent urinary signals for non-invasive early diagnosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


