美国气溶胶酸度年代际变化趋势的关键驱动过程
IF 6.7
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 1
摘要
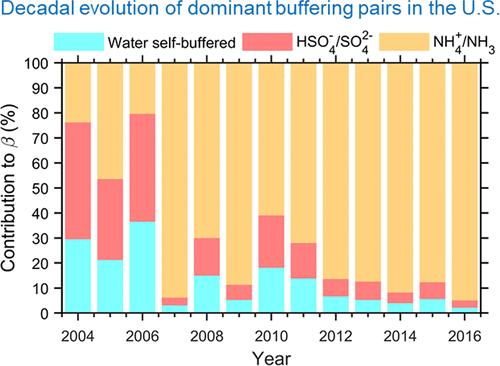
酸度是决定大气中水相物理和化学过程的一个重要参数,它强烈影响气溶胶对气候、生态和健康的影响。传统上认为,气溶胶的酸度随着大气酸性物质(SO2、NOx等)的排放而增加,而随着碱性物质(NH3、粉尘等)的排放而减少。然而,在美国东南部长达十年的观测似乎不同意这一假设:虽然NH3与SO2的排放量增加了三倍以上,但预测的气溶胶酸度是稳定的,而且观测到的颗粒相氨与硫酸盐的比例甚至在下降。在这里,我们用最近提出的多相缓冲理论来研究这个问题。我们表明,从历史上看,该地区气溶胶酸度的主要驱动因素发生了转变。在~ 2008年之前的贫氨条件下,酸度由HSO4 - /SO42 -缓冲和水的自缓冲作用控制。在富氨条件下,~ 2008年后,气溶胶酸度主要由NH4+/NH3缓冲。在研究期间,有机酸的缓冲作用可以忽略不计。此外,观察到的氨硫酸盐比的下降是由于非挥发性阳离子的重要性增加,特别是在~ 2014年之后。我们预测,直到2050年,气溶胶仍将处于氨缓冲状态,而在美国东南部,硝酸盐将大部分(98%)保持在气相中本文章由计算机程序翻译,如有差异,请以英文原文为准。
Revisiting the Key Driving Processes of the Decadal Trend of Aerosol Acidity in the U.S
Acidity is one essential parameter in determining the aqueous phase physical and chemical processes in the atmosphere and strongly influences the climate, ecological, and health effects of aerosols. Traditionally, aerosol acidity is thought to increase with emissions of atmospheric acidic substances (SO2, NOx, etc.) and decrease with that of alkaline ones (NH3, dust, etc.). However, decade-long observations in southeastern U.S. seem to disagree with this hypothesis: while the emissions of NH3 versus SO2 enhanced by over three times, the predicted aerosol acidity is stable, and the observed particle-phase ammonium-to-sulfate ratio is even decreasing. Here, we investigated into this issue with the recently proposed multiphase buffer theory. We show that historically, there is a transition in the dominant drivers of aerosol acidity in this region. Under the ammonia-poor conditions before ∼2008, the acidity is governed by HSO4–/SO42– buffering and the water self-buffering effect. Under the ammonia-rich conditions after ∼2008, aerosol acidity is mainly buffered by NH4+/NH3. Buffering from the organic acids is negligible in the investigated period. In addition, the observed decrease in ammonium-to-sulfate ratio is due to the increased importance of non-volatile cations, especially after ∼2014. We predict that until ∼2050, the aerosols will remain in the ammonia-buffered regime, and the nitrate will remain largely (>98%) in the gas phase in southeastern U.S.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Environmental Au
环境科学-
CiteScore
7.10
自引率
0.00%
发文量
0
期刊介绍:
ACS Environmental Au is an open access journal which publishes experimental research and theoretical results in all aspects of environmental science and technology both pure and applied. Short letters comprehensive articles reviews and perspectives are welcome in the following areas:Alternative EnergyAnthropogenic Impacts on Atmosphere Soil or WaterBiogeochemical CyclingBiomass or Wastes as ResourcesContaminants in Aquatic and Terrestrial EnvironmentsEnvironmental Data ScienceEcotoxicology and Public HealthEnergy and ClimateEnvironmental Modeling Processes and Measurement Methods and TechnologiesEnvironmental Nanotechnology and BiotechnologyGreen ChemistryGreen Manufacturing and EngineeringRisk assessment Regulatory Frameworks and Life-Cycle AssessmentsTreatment and Resource Recovery and Waste Management
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


