Kymriah®(tisagenlcleucel)-首个获批CAR-T疗法的临床开发历程概述。
IF 4.8
4区 医学
Human Vaccines & Immunotherapeutics
Pub Date : 2023-12-31
Epub Date: 2023-05-15
DOI:10.1080/21645515.2023.2210046
引用次数: 0
摘要
细胞和基因疗法的出现极大地改变了肿瘤学和其他治疗领域的治疗模式。Kymriah®(tisagenleucel)是一种CD19导向的转基因自体T细胞免疫疗法,目前已在主要市场被批准用于治疗复发/难治性(r/r)儿童和年轻成人急性淋巴细胞白血病、r/r弥漫性大B细胞淋巴瘤和r/r滤泡性淋巴瘤。本文对tisagenleckel的临床开发历程进行了高水平的概述,包括其疗效和安全性考虑。本文章由计算机程序翻译,如有差异,请以英文原文为准。
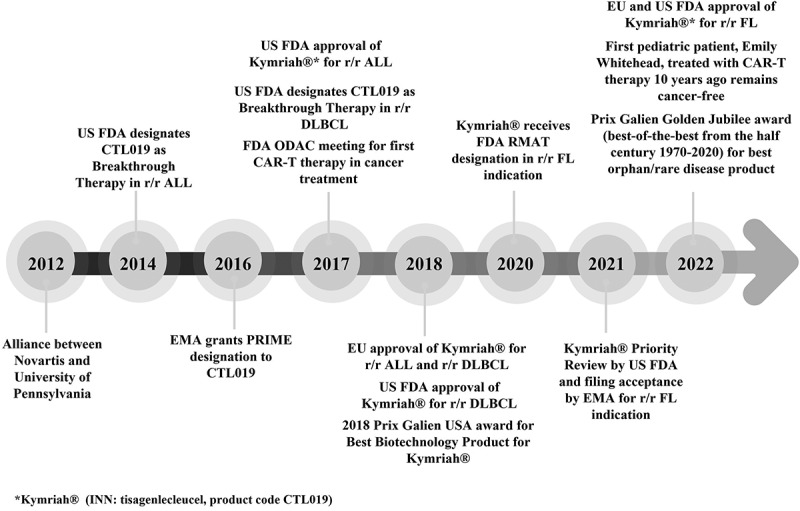
Kymriah® (tisagenlecleucel) - An overview of the clinical development journey of the first approved CAR-T therapy.
The emergence of cell and gene therapies has dramatically changed the treatment paradigm in oncology and other therapeutic areas. Kymriah® (tisagenlecleucel), a CD19-directed genetically modified autologous T-cell immunotherapy, is currently approved in major markets for the treatment of relapsed/refractory (r/r) pediatric and young adult acute lymphoblastic leukemia, r/r diffuse large B-cell lymphoma, and r/r follicular lymphoma. This article presents a high-level overview of the clinical development journey of tisagenlecleucel, including its efficacy outcomes and safety considerations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Human Vaccines & Immunotherapeutics
BIOTECHNOLOGY & APPLIED MICROBIOLOGY-IMMUNOLOGY
自引率
8.30%
发文量
0
审稿时长
1 months
期刊介绍:
(formerly Human Vaccines; issn 1554-8619)
Vaccine research and development is extending its reach beyond the prevention of bacterial or viral diseases. There are experimental vaccines for immunotherapeutic purposes and for applications outside of infectious diseases, in diverse fields such as cancer, autoimmunity, allergy, Alzheimer’s and addiction. Many of these vaccines and immunotherapeutics should become available in the next two decades, with consequent benefit for human health. Continued advancement in this field will benefit from a forum that can (A) help to promote interest by keeping investigators updated, and (B) enable an exchange of ideas regarding the latest progress in the many topics pertaining to vaccines and immunotherapeutics.
Human Vaccines & Immunotherapeutics provides such a forum. It is published monthly in a format that is accessible to a wide international audience in the academic, industrial and public sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


