体细胞变异报告定制三级分析平台的实现。
IF 2.6
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
肿瘤精准医学需要对实体瘤和血液恶性肿瘤分子谱中识别的变异进行评估。这包括分析前和分析后质量指标的评估、变异解释、分类和分级,以及与临床意义(如FDA批准的药物和临床试验)的关联,最后是综合报告。这项研究记录了我们在定制和实现软件平台方面的经验,该软件平台促进了有效报告体细胞变异的这些需求。本文章由计算机程序翻译,如有差异,请以英文原文为准。

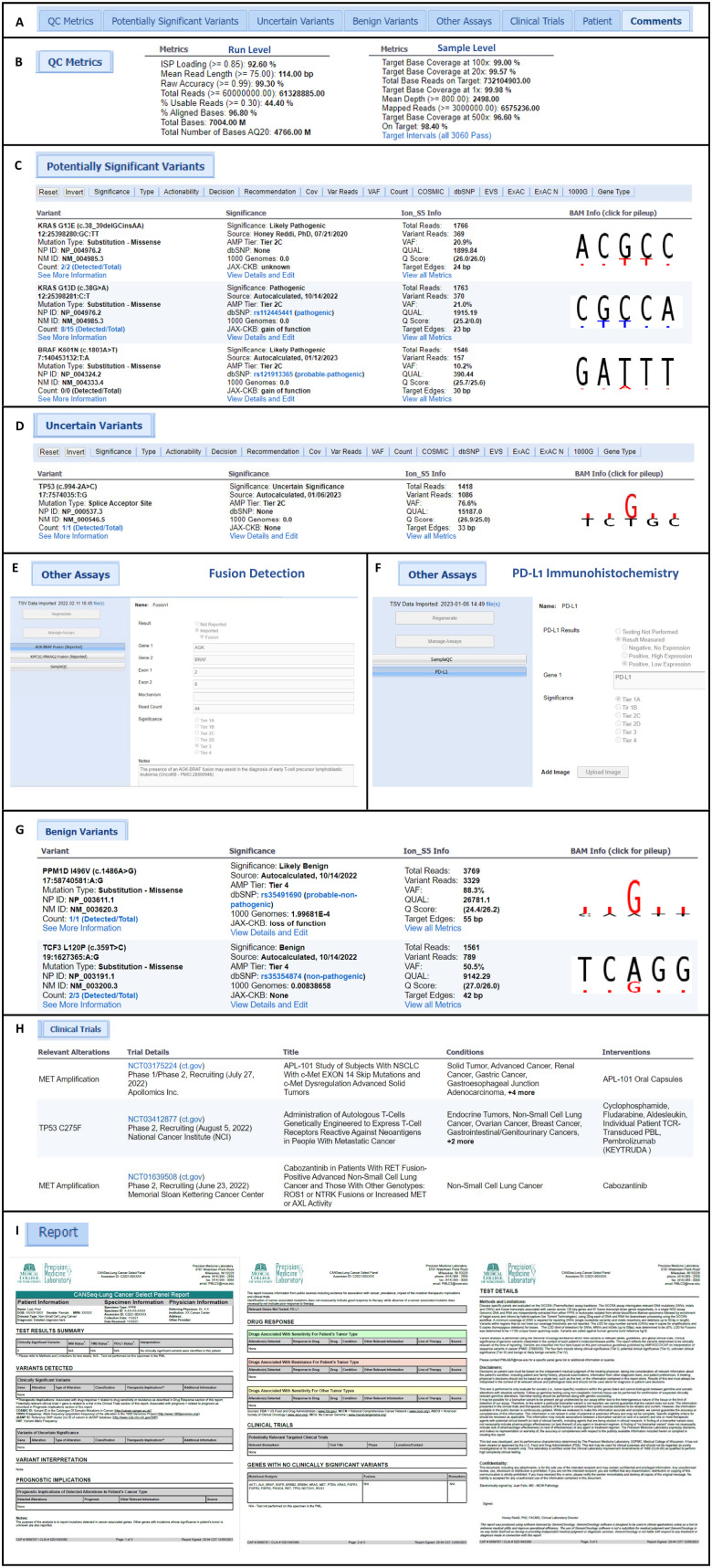
Implementation of a Customized Tertiary Analysis Platform for the Reporting of Somatic Variants.
Precision medicine for oncology requires the evaluation of variants identified in molecular profiling of solid tumors and hematologic malignancies. This includes evaluation of pre-analytical and postanalytical quality metrics, variant interpretation, classification, and tiering as outlined in established guidelines, association with clinical significance such as FDA approved drugs and clinical trials, and finally comprehensive reporting. This study documents our experience with the customization and implementation of a software platform that facilitates these requirements for effective reporting of somatic variants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomarker Insights
MEDICINE, RESEARCH & EXPERIMENTAL-
CiteScore
6.00
自引率
0.00%
发文量
26
审稿时长
8 weeks
期刊介绍:
An open access, peer reviewed electronic journal that covers all aspects of biomarker research and clinical applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


