一项前瞻性随机试验:4mg·kg糖美德用于同时进行胰肾移植的安全性和有效性。
IF 1.4
4区 医学
Q3 SURGERY
引用次数: 1
摘要
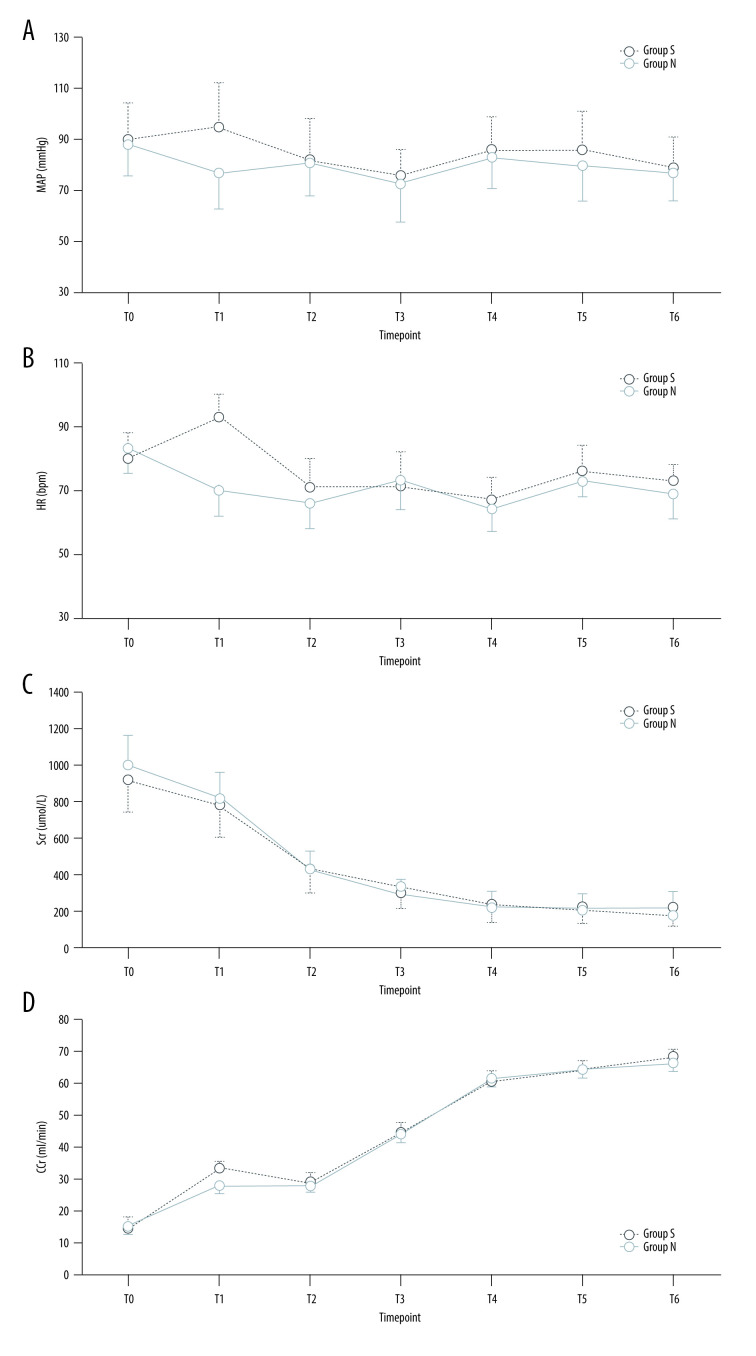
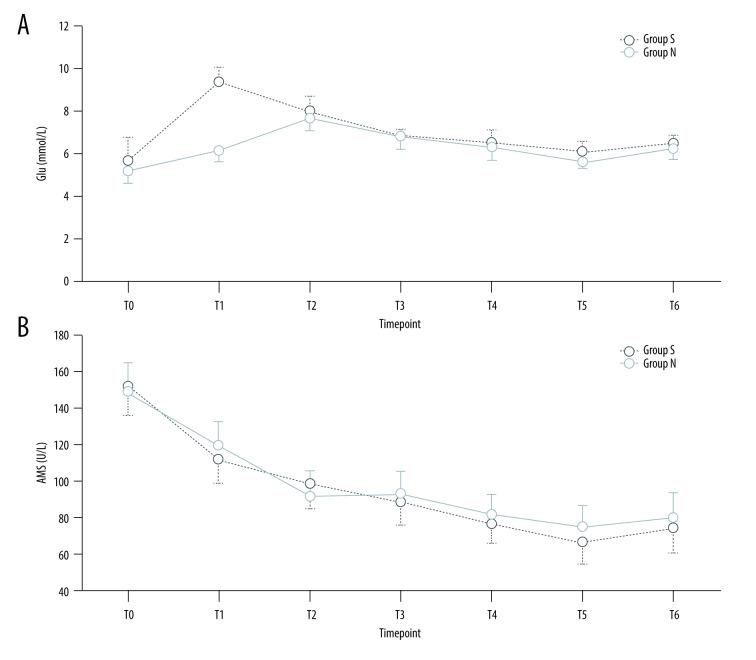
同时胰肾移植(SPK)是一项耗时且重要的外科手术,它可以提供一种生理手段来实现正常血糖并使患者免于透析。sugammadex的潜在临床益处包括快速和可预测的反向深度神经肌肉阻断(NMB),但sugammadex是否影响SPK移植物的功能尚不确定。材料与方法对48例患者进行了研究,并使用sugammadex (n=24)或新斯的明(n=24)逆转了深部NMB。安全性变量包括血清肌酐(Scr)、肌酐清除率(CCr)、血清淀粉酶(AMS)、血糖(Glu)、平均动脉压(MAP)和心率(HR)。次要结局是从预定时间给药糖马德/新斯的明到TOF比恢复到0.7和0.9的时间,以及急性后肺部并发症。结果T2-6时Scr显著低于t2 -1时(P0.05)。T1时,S组MAP、HR、Glu均高于N组(P本文章由计算机程序翻译,如有差异,请以英文原文为准。
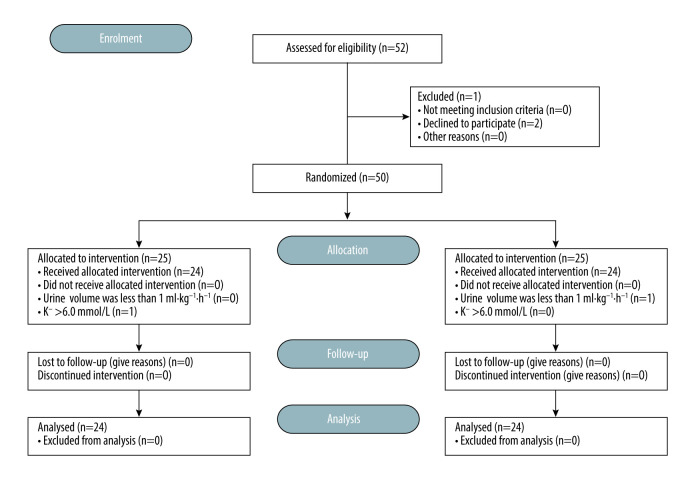
Safety and Efficacy of 4 mg·kg⁻¹ Sugammadex for Simultaneous Pancreas-Kidney Transplantation Recipients: A Prospective Randomized Trial.
Background Simultaneous pancreas-kidney transplantation (SPK) is a time-consuming and important surgical procedure, which can provide a physiological mean of achieving normoglycemia and render patients free of dialysis. The potential clinical benefits of sugammadex include fast and predictable reverse deep neuromuscular blockade (NMB), but whether sugammadex affects the function of SPK grafts is uncertain. Material/Methods Forty-eight patients were studied and reversed deep NMB with either sugammadex (n=24) or neostigmine (n=24). The safety variables included serum creatinine (Scr), creatinine clearance rate (CCr), serum amylase (AMS), blood glucose (Glu), mean arterial pressure (MAP), and heart rate (HR). Secondary outcomes were time from administration of sugammadex/neostigmine at the scheduled time to recovery of a TOF ratio to 0.7 and 0.9, and post-acute pulmonary complications. Results Scr at T2–6 was significantly lower than that at T0–1 (P<0.01), while CCr was higher (P<0.05). Between the 2 groups, Scr, CCr, and AMS were similar at the same timepoints (P>0.05). MAP, HR, and Glu were higher in group S than in group N at T1 (P<0.05). The recovery time of TOF=0.7 was 3 (2.4–4.2) min for group S and 12.1 (10.2–15.9) min for group N (P<0.001), and recovery time to TOFr ≥0.9 was 4.8 (3.6–7.1) min for group S and 23.5 (19.8–30.8) in group S. Compared to group N, group S had lower risk for post-acute pulmonary complications: supplemental oxygen requirements 0 vs 4 (16.7%), pulmonary atelectasis 0 vs 2 (0.83%), pneumonia 1 (4.2%) vs 3 (12.5%), and hypoxemia 1 (4.2%) vs 4 (16.7%). Conclusions Sugammadex administration is safe and effective for SPK transplantation recipients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
2.50
自引率
0.00%
发文量
79
审稿时长
>12 weeks
期刊介绍:
Annals of Transplantation is one of the fast-developing journals open to all scientists and fields of transplant medicine and related research. The journal is published quarterly and provides extensive coverage of the most important advances in transplantation.
Using an electronic on-line submission and peer review tracking system, Annals of Transplantation is committed to rapid review and publication. The average time to first decision is around 3-4 weeks. Time to publication of accepted manuscripts continues to be shortened, with the Editorial team committed to a goal of 3 months from acceptance to publication.
Expert reseachers and clinicians from around the world contribute original Articles, Review Papers, Case Reports and Special Reports in every pertinent specialty, providing a lot of arguments for discussion of exciting developments and controversies in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


