药物基因组学:现状与未来展望
IF 39.1
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 21
摘要
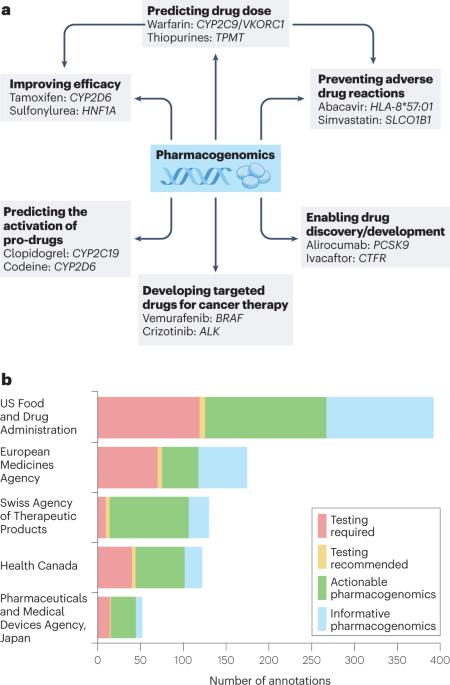
无论是疗效还是安全性,药物反应的个体间差异都很常见,而且随着需要治疗的老年人口不断增加,这一问题可能会在全球范围内日益严重。造成个体间差异的原因包括基因组因素,这一研究领域被称为药物基因组学。随着基因分型技术的普及和成本的降低,将药物基因组学应用于临床实践--被广泛认为是基因组医学主流化的第一步--目前已成为全球许多国家的关注焦点。然而,实施的主要挑战在于将药物基因组学应用于医疗保健系统,包括修改当前的临床路径,以及医疗保健人员在药物基因组学方面存在的巨大知识缺口。药物基因组学还可以在更广泛的意义上用于药物发现和开发,越来越多的证据表明,基因组学定义的靶点在临床开发中的成功率更高。在这篇综述中,Munir Pirmohamed 概述了药物基因组学领域的现状,并举例说明了与临床相关的药物基因关联,然后概述了将药物基因组学应用于临床实践所需的步骤。该书还探讨了药物基因组学在药物发现和开发中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pharmacogenomics: current status and future perspectives
Inter-individual variability in drug response, be it efficacy or safety, is common and likely to become an increasing problem globally given the growing elderly population requiring treatment. Reasons for this inter-individual variability include genomic factors, an area of study called pharmacogenomics. With genotyping technologies now widely available and decreasing in cost, implementing pharmacogenomics into clinical practice — widely regarded as one of the initial steps in mainstreaming genomic medicine — is currently a focus in many countries worldwide. However, major challenges of implementation lie at the point of delivery into health-care systems, including the modification of current clinical pathways coupled with a massive knowledge gap in pharmacogenomics in the health-care workforce. Pharmacogenomics can also be used in a broader sense for drug discovery and development, with increasing evidence suggesting that genomically defined targets have an increased success rate during clinical development. In this Review, Munir Pirmohamed provides an overview of the current state of the pharmacogenomics field, using examples of clinically relevant drug–gene associations, before outlining the steps needed for implementation of pharmacogenomics into clinical practice. The role of pharmacogenomics in drug discovery and development is also considered.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Reviews Genetics
生物-遗传学
CiteScore
57.40
自引率
0.50%
发文量
113
审稿时长
6-12 weeks
期刊介绍:
At Nature Reviews Genetics, our goal is to be the leading source of reviews and commentaries for the scientific communities we serve. We are dedicated to publishing authoritative articles that are easily accessible to our readers. We believe in enhancing our articles with clear and understandable figures, tables, and other display items. Our aim is to provide an unparalleled service to authors, referees, and readers, and we are committed to maximizing the usefulness and impact of each article we publish.
Within our journal, we publish a range of content including Research Highlights, Comments, Reviews, and Perspectives that are relevant to geneticists and genomicists. With our broad scope, we ensure that the articles we publish reach the widest possible audience.
As part of the Nature Reviews portfolio of journals, we strive to uphold the high standards and reputation associated with this esteemed collection of publications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


